Global distribution, public health and clinical impact of the protozoan pathogen cryptosporidium
- PMID: 20706669
- PMCID: PMC2913630
- DOI: 10.1155/2010/753512
Global distribution, public health and clinical impact of the protozoan pathogen cryptosporidium
Abstract
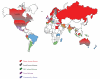
Cryptosporidium spp. are coccidians, oocysts-forming apicomplexan protozoa, which complete their life cycle both in humans and animals, through zoonotic and anthroponotic transmission, causing cryptosporidiosis. The global burden of this disease is still underascertained, due to a conundrum transmission modality, only partially unveiled, and on a plethora of detection systems still inadequate or only partially applied for worldwide surveillance. In children, cryptosporidiosis encumber is even less recorded and often misidentified due to physiological reasons such as early-age unpaired immunological response. Furthermore, malnutrition in underdeveloped countries or clinical underestimation of protozoan etiology in developed countries contribute to the underestimation of the worldwide burden. Principal key indicators of the parasite distribution were associated to environmental (e.g., geographic and temporal clusters, etc.) and host determinants of the infection (e.g., age, immunological status, travels, community behaviours). The distribution was geographically mapped to provide an updated picture of the global parasite ecosystems. The present paper aims to provide, by a critical analysis of existing literature, a link between observational epidemiological records and new insights on public health, and diagnostic and clinical impact of cryptosporidiosis.
Figures

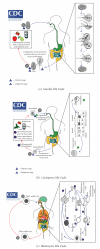

References
-
- Goodgame RW. Understanding intestinal spore-forming protozoa: cryptosporidia, microsporidia, Isospora, and Cyclospora. Annals of Internal Medicine. 1996;124(4):429–441. - PubMed
-
- Eckmann L. Small bowel infections. Current Opinion in Gastroenterology. 2002;18(2):197–202. - PubMed
-
- Pierce KK, Kirkpatrick BD. Update on human infections caused by intestinal protozoa. Current Opinion in Gastroenterology. 2009;25(1):12–17. - PubMed
-
- World Health Organisation. Tech. Rep. WHO/CDS/IPI/92.2. Geneva, Switzerland: World Health Organisation; 1992. WHO/PAHO informal consultation on intestinal protozoal infections.
-
- Chen X-M, Keithly JS, Paya CV, LaRusso NF. Cryptosporidiosis. The New England Journal of Medicine. 2002;346(22):1723–1731. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

