Integrating individual functional moieties of CXCL10 and CXCL11 into a novel chimeric chemokine leads to synergistic antitumor effects: a strategy for chemokine-based multi-target-directed cancer therapy
- PMID: 20706716
- PMCID: PMC11030099
- DOI: 10.1007/s00262-010-0901-6
Integrating individual functional moieties of CXCL10 and CXCL11 into a novel chimeric chemokine leads to synergistic antitumor effects: a strategy for chemokine-based multi-target-directed cancer therapy
Abstract
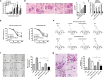
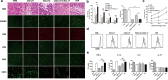
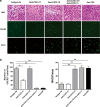
The complexity of tumor biology necessitates a multimodality approach that targets different aspects of tumor environment in order to generate the greatest benefit. IFN-inducible T cell alpha chemoattractant (ITAC)/CXCL11 and IFN-inducible protein 10 (IP10)/CXCL10 could exert antitumor effects with functional specificity and thus emerge as attractive candidates for combinatorial strategy. Disappointedly, a synergistic antitumor effect could not be observed when CXCL10 and CXCL11 were pooled together. In this regard, we seek to improve antitumor efficacy by integrating their individual functional moieties into a chemokine chimeric molecule, designated ITIP, which was engineered by substituting the N-terminal and N-loop region of CXCL10 with those of CXCL11. The functional properties of ITIP were determined by chemotaxis and angiogenesis assays. The antitumor efficacy was tested in murine CT26 colon carcinoma, 4T1 mammary carcinoma and 3LL lung carcinoma. Here we showed that ITIP not only exhibited respective functional superiority but strikingly promoted regression of established tumors and remarkably prolonged survival of mice compared with its parent chemokines, either alone or in combination. The chemokine chimera induced an augmented anti-tumor immunity and a marked decrease in tumor vasculature. Antibody neutralization studies indicated that CXCL10 and CXCL11 moieties of ITIP were responsible for anti-angiogenesis and chemotaxis in antitumor response, respectively. These results indicated that integrating individual functional moieties of CXCL10 and CXCL11 into a chimeric chemokine could lead to a synergistic antitumor effect. Thus, this integration strategy holds promise for chemokine-based multiple targeted therapy of cancer.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
The CXCR3 targeting chemokine CXCL11 has potent antitumor activity in vivo involving attraction of CD8+ T lymphocytes but not inhibition of angiogenesis.J Immunother. 2005 Jul-Aug;28(4):343-51. doi: 10.1097/01.cji.0000165355.26795.27. J Immunother. 2005. PMID: 16000952
-
Cross reactivity of three T cell attracting murine chemokines stimulating the CXC chemokine receptor CXCR3 and their induction in cultured cells and during allograft rejection.Eur J Immunol. 2001 Aug;31(8):2521-7. doi: 10.1002/1521-4141(200108)31:8<2521::aid-immu2521>3.0.co;2-q. Eur J Immunol. 2001. PMID: 11500837
-
Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-γ in experimental dry eye.J Immunol. 2014 Nov 15;193(10):5264-72. doi: 10.4049/jimmunol.1400016. Epub 2014 Oct 6. J Immunol. 2014. PMID: 25288568 Free PMC article.
-
CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy.Cancer Treat Rev. 2018 Feb;63:40-47. doi: 10.1016/j.ctrv.2017.11.007. Epub 2017 Nov 26. Cancer Treat Rev. 2018. PMID: 29207310 Free PMC article. Review.
-
CXCR3 Ligands in Cancer and Autoimmunity, Chemoattraction of Effector T Cells, and Beyond.Front Immunol. 2020 May 29;11:976. doi: 10.3389/fimmu.2020.00976. eCollection 2020. Front Immunol. 2020. PMID: 32547545 Free PMC article. Review.
Cited by
-
Enhancer of zeste homolog 2 enhances the migration and chemotaxis of dental mesenchymal stem cells.J Int Med Res. 2020 Jan;48(1):300060519882149. doi: 10.1177/0300060519882149. Epub 2019 Oct 23. J Int Med Res. 2020. PMID: 31642363 Free PMC article.
-
Down-regulation of CXCL11 inhibits colorectal cancer cell growth and epithelial-mesenchymal transition.Onco Targets Ther. 2018 Oct 23;11:7333-7343. doi: 10.2147/OTT.S167872. eCollection 2018. Onco Targets Ther. 2018. PMID: 30425523 Free PMC article.
-
CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications.Cytokine Growth Factor Rev. 2011 Jun;22(3):121-30. doi: 10.1016/j.cytogfr.2011.06.001. Epub 2011 Jul 29. Cytokine Growth Factor Rev. 2011. PMID: 21802343 Free PMC article. Review.
-
The role of CXCR3 and its ligands in cancer.Front Oncol. 2022 Nov 21;12:1022688. doi: 10.3389/fonc.2022.1022688. eCollection 2022. Front Oncol. 2022. PMID: 36479091 Free PMC article. Review.
-
Role of Chemokines in Non-Small Cell Lung Cancer: Angiogenesis and Inflammation.J Cancer. 2015 Aug 7;6(10):938-52. doi: 10.7150/jca.12286. eCollection 2015. J Cancer. 2015. PMID: 26316890 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

