First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience
- PMID: 20706757
- PMCID: PMC2936684
- DOI: 10.1007/s10143-010-0280-7
First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience
Abstract
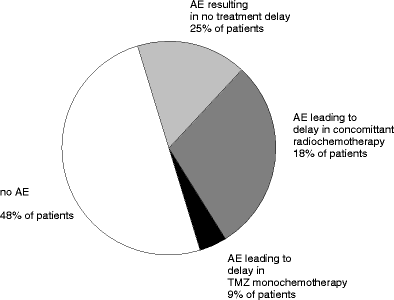
Randomized phase III trials have shown significant improvement of survival 1, 2, and 3 years after implantation of 1,3-bis (2-chloroethyl)-1-nitrosourea (BCNU) wafers for patients with newly diagnosed malignant glioma. But these studies and subsequent non-phase III studies have also shown risks associated with local chemotherapy within the central nervous system. The introduction of concomitant radiochemotherapy with temozolomide (TMZ) has later demonstrated a survival benefit in a phase III trial and has become the current treatment standard for newly diagnosed malignant glioma patients. Lately, this has resulted in clinical protocols combining local chemotherapy with BCNU wafers and concomitant radiochemotherapy with TMZ although this may carry the risk of increased toxicity. We have compiled the treatment experience of seven neurosurgical centers using implantation of carmustine wafers at primary surgery followed by 6 weeks of radiation therapy (59-60 Gy) and 75 mg/m(2)/day TMZ in patients with newly diagnosed glioblastoma followed by TMZ monochemotherapy. We have retrospectively analyzed the postoperative clinical course, occurrence and severity of adverse events, progression-free interval, and overall survival in 44 patients with newly diagnosed glioblastoma multiforme. All patients received multimodal treatment including tumor resection, BCNU wafer implantation, and concomitant radiochemotherapy. Of 44 patients (mean age 59 ± 10.8 years) with glioblastoma who received Gliadel wafer at primary surgery, 28 patients (64%) had died, 16 patients (36%) were alive, and 15 patients showed no evidence of clinical or radiographic progression after a median follow-up of 15.6 months. At time of analysis of adverse events in this patient population, the median overall survival was 12.7 months and median progression-free survival was 7.0 months. Surgical, neurological, and medical adverse events were analyzed. Twenty-three patients (52%) experienced adverse events of any kind including complications that did not require treatment. Nineteen patients (43%) experienced grade 3 or grade 4 adverse events. Surgical complications included cerebral edema, healing abnormalities, cerebral spinal fluid leakage, meningitis, intracranial abscess, and hydrocephalus. Neurological adverse events included newly diagnosed seizures, alteration of mental status, and new neurological deficits. Medical complications were thromboembolic events (thrombosis, pulmonary embolism) and hematotoxicity. Combination of both treatment strategies, local chemotherapy with BCNU wafer and concomitant radiochemotherapy, appears attractive in aggressive multimodal treatment schedules and may utilize the sensitizing effect of TMZ and carmustine on MGMT and AGT on their respective drug resistance genes. Our data demonstrate that combination of local chemotherapy and concomitant radiochemotherapy carries a significant risk of toxicity that currently appears underestimated. Adverse events observed in this study appear similar to complication rates published in the phase III trials for BCNU wafer implantation followed by radiation therapy alone, but further add the toxicity of concomitant radiochemotherapy with systemic TMZ. Save use of a combined approach will require specific prevention strategies for multimodal treatments.
Conflict of interest statement
None.
Figures
Similar articles
-
MGMT promoter methylation status and prognosis of patients with primary or recurrent glioblastoma treated with carmustine wafers.Br J Neurosurg. 2013 Dec;27(6):772-8. doi: 10.3109/02688697.2013.791664. Epub 2013 May 11. Br J Neurosurg. 2013. PMID: 23662801 Clinical Trial.
-
Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review.World J Surg Oncol. 2016 Aug 24;14(1):225. doi: 10.1186/s12957-016-0975-5. World J Surg Oncol. 2016. PMID: 27557526 Free PMC article.
-
Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme.J Neurosurg. 2009 Mar;110(3):583-8. doi: 10.3171/2008.5.17557. J Neurosurg. 2009. PMID: 19046047 Free PMC article.
-
Advantages and Disadvantages of Combined Chemotherapy with Carmustine Wafer and Bevacizumab in Patients with Newly Diagnosed Glioblastoma: A Single-Institutional Experience.World Neurosurg. 2018 May;113:e508-e514. doi: 10.1016/j.wneu.2018.02.070. Epub 2018 Feb 21. World Neurosurg. 2018. PMID: 29476996
-
Polymeric drug delivery for the treatment of glioblastoma.Neuro Oncol. 2015 Mar;17 Suppl 2(Suppl 2):ii9-ii23. doi: 10.1093/neuonc/nou360. Neuro Oncol. 2015. PMID: 25746091 Free PMC article. Review.
Cited by
-
Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: a meta-analysis.J Neurooncol. 2015 Apr;122(2):367-82. doi: 10.1007/s11060-015-1724-2. Epub 2015 Jan 29. J Neurooncol. 2015. PMID: 25630625 Free PMC article.
-
Advanced materials and processing for drug delivery: the past and the future.Adv Drug Deliv Rev. 2013 Jan;65(1):104-20. doi: 10.1016/j.addr.2012.10.003. Epub 2012 Oct 23. Adv Drug Deliv Rev. 2013. PMID: 23088863 Free PMC article. Review.
-
Polifeprosan 20, 3.85% carmustine slow-release wafer in malignant glioma: evidence for role in era of standard adjuvant temozolomide.Core Evid. 2012;7:115-30. doi: 10.2147/CE.S23244. Epub 2012 Oct 26. Core Evid. 2012. PMID: 23118709 Free PMC article.
-
Overexpression of Nrf2 attenuates Carmustine-induced cytotoxicity in U87MG human glioma cells.BMC Cancer. 2015 Mar 13;15:118. doi: 10.1186/s12885-015-1134-z. BMC Cancer. 2015. PMID: 25851054 Free PMC article.
-
New developments in surgery of malignant gliomas.Radiol Oncol. 2011 Sep;45(3):159-65. doi: 10.2478/v10019-011-0018-3. Epub 2011 Jul 20. Radiol Oncol. 2011. PMID: 22933950 Free PMC article.
References
-
- Asher A (2007) Prospective analysis of temozolomide as adjuvant to Gliadel and radiation in newly diagnosed malignant glioma. Abstract presented at the Annual Meeting of the American Association of Neurological Surgeons, Washington, DC 2007
-
- Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, Black K, Sisti M, Brem S, Mohr G, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/S0140-6736(95)90755-6. - DOI - PubMed
-
- Cancer Therapy Evaluation Program (2006) Common terminology criteria for adverse events, version 3.0, DCTD, NCI, NIH, DHHS, March 31, 2003 (http://ctep.cancer.gov). Publish Date: August 9
-
- Fung LK, Ewend MG, Sills A, Sipos EP, Thompson R, Watts M, Colvin OM, Brem H, Saltzman WM. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58:672–684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous