The inhaled glucocorticoid fluticasone propionate efficiently inactivates cytochrome P450 3A5, a predominant lung P450 enzyme
- PMID: 20707410
- PMCID: PMC2924751
- DOI: 10.1021/tx100124k
The inhaled glucocorticoid fluticasone propionate efficiently inactivates cytochrome P450 3A5, a predominant lung P450 enzyme
Abstract
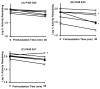
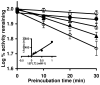
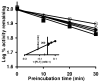
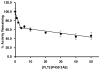
Inhaled glucocorticoid (GC) therapy is a vital part of the management of chronic asthma. GCs are metabolized by members of the cytochrome P450 3A family in both liver and lung, but the enzymes are differentially expressed. Selective inhibition of one or more P450 3A enzymes could substantially modify target and systemic concentrations of GCs. In this study, we have evaluated the mechanism-based inactivation of P450 3A4, 3A5, and 3A7 enzymes by GCs. Among the five major inhaled GCs approved for clinical use in the United States, fluticasone propionate (FLT) was the most potent mechanism-based inactivator of P450 3A5, the predominant P450 enzyme in the lung. FLT inactivated P450 3A5 in a time- and concentration-dependent manner with K(I), k(inact), and partition ratio of 16 muM, 0.027 min(-1), and 3, respectively. In contrast, FLT minimally inactivated P450 3A4 and did not inactivate 3A7, even with a concentration of 100 muM. The inactivation of P450 3A5 by FLT was irreversible because dialysis did not restore enzyme activity. In addition, the exogenous nucleophilic scavenger GSH did not attenuate inactivation. The prosthetic heme of P450 3A5 was not modified by FLT. The loss of P450 3A5 activity in lung cells could substantially decrease the metabolism of FLT, which would increase the effective FLT concentration at its target site, the respiratory epithelium. Also, inactivation of lung P450 3A5 could increase the absorption of inhaled FLT, which could lead to high systemic concentrations and adverse effects, such as life-threatening adrenal crises or cataracts that have been documented in children receiving high doses of inhaled GCs.
Figures
References
-
- Undem BJ. Pharmacotherapy of Asthma. In: Brunton LL, Lazo JS, Parker KL, editors. The Pharmacological Basis of Therapeutics. The McGraw-Hill Companies; New York: 2005. pp. 717–736.
-
- Leung DYM, Bloom JW. Update on Glucocorticoid Action and Resistance. Journal of allergy and Clinical Immunology. 2003;111:3–22. - PubMed
-
- Mjaanes CM, Whelan GJ, Szefler SJ. Corticosteroid therapy in Asthma: Predictors of Responsiveness. Clinics in Chest Medicine. 2006;27:119–132. - PubMed
-
- Zhang JY, Wang Y, Prakash C. Xenobiotic-metabolizing enzymes in human lung. Current drug metabolism. 2006;7:939–948. - PubMed
-
- Bernauer U, Heinrich-Hirsch B, Tonnies M, Peter-Matthias W, Gundert-Remy U. Characterisation of the xenobiotic-metabolizing Cytochrome P450 expression pattern in human lung tissue by immunochemical and activity determination. Toxicol Lett. 2006;164:278–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

