Evidence for rosettes as an unrecognized stage in the life cycle of Leishmania parasites
- PMID: 20707828
- PMCID: PMC3086735
- DOI: 10.1111/j.1550-7408.2010.00496.x
Evidence for rosettes as an unrecognized stage in the life cycle of Leishmania parasites
Abstract
Leishmania parasites, which afflict 12 million people in 88 countries, exist as promastigotes transmitted by insect vectors and as amastigotes residing in mammalian macrophages. Promastigote cells arranged in rosettes have also been described but universally disregarded as a distinct stage in the life cycle. We present evidence that only rosettes of Leishmania major promastigotes express cell surface poly-alpha2,8 N-acetyl neuraminic acid (PSA) and PSA containing de-N-acetyl neuraminic acid (NeuPSA). Expression of rosette-specific PSA antigens was mosaic, with individual promastigotes expressing PSA, NeuPSA or both. A 50 kDa protein was detected by Western blot analysis of a detergent-insoluble cell fraction with both PSA and NeuPSA-reactive antibodies. Frequencies of rosette formation as well as cell surface PSA/NeuPSA expression were temperature dependent. Rosettes also engaged in an unusual swarming behavior, congregating into extended clusters. Distinct structures resembling cellular fusion bodies were formed in and released from rosettes. The results indicate that rosettes are an unrecognized stage in the life cycle of Leishmania. We hypothesize that rosettes initiate mating in Leishmania during which PSA/NeuPSA expression plays an important role. Recognizing rosettes as a distinct form of the Leishmania life cycle opens new possibilities for treatment or prevention of disease and, possibly, in vitro genetic recombination without passage of cells through insect vectors.
Figures





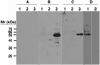

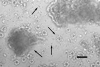


References
-
- Abramoff MD, Magelhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Internl. 2004;11:36–42.
-
- Adair WS, Monk BC, Cohen R, Hwang C, Goodenough UW. Sexual agglutinins from the Chlamydomonas flagellar membrane. Partial purification and characterization. J. Biol. Chem. 1982;257:4593–4602. - PubMed
-
- Angata T, Varki A. Chemical diversity in the sialic acids and related alpha-keto acids: An evolutionary perspective. Chem. Rev. 2002;102:439–469. - PubMed
-
- Bastien P, Blaineau C, Pages M. Leishmania: sex, lies and karyotype. Parasitol. Today. 1992;8:174–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

