Wallerian-like axonal degeneration in the optic nerve after excitotoxic retinal insult: an ultrastructural study
- PMID: 20707883
- PMCID: PMC2930628
- DOI: 10.1186/1471-2202-11-97
Wallerian-like axonal degeneration in the optic nerve after excitotoxic retinal insult: an ultrastructural study
Abstract
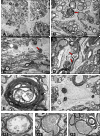
Background: Excitotoxicity is involved in the pathogenesis of a number neurodegenerative diseases, and axonopathy is an early feature in several of these disorders. In models of excitotoxicity-associated neurological disease, an excitotoxin delivered to the central nervous system (CNS), could trigger neuronal death not only in the somatodendritic region, but also in the axonal region, via oligodendrocyte N-methyl-D-aspartate (NMDA) receptors. The retina and optic nerve, as approachable regions of the brain, provide a unique anatomical substrate to investigate the "downstream" effect of isolated excitotoxic perikaryal injury on central nervous system (CNS) axons, potentially providing information about the pathogenesis of the axonopathy in clinical neurological disorders.Herein, we provide ultrastructural information about the retinal ganglion cell (RGC) somata and their axons, both unmyelinated and myelinated, after NMDA-induced retinal injury. Male Sprague-Dawley rats were killed at 0 h, 24 h, 72 h and 7 days after injecting 20 nM NMDA into the vitreous chamber of the left eye (n = 8 in each group). Saline-injected right eyes served as controls. After perfusion fixation, dissection, resin-embedding and staining, ultrathin sections of eyes and proximal (intraorbital) and distal (intracranial) optic nerve segments were evaluated by transmission electron tomography (TEM).
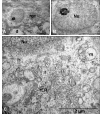
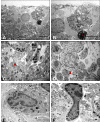
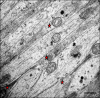
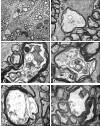
Results: TEM demonstrated features of necrosis in RGCs: mitochondrial and endoplasmic reticulum swelling, disintegration of polyribosomes, rupture of membranous organelle and formation of myelin bodies. Ultrastructural damage in the optic nerve mimicked the changes of Wallerian degeneration; early nodal/paranodal disturbances were followed by the appearance of three major morphological variants: dark degeneration, watery degeneration and demyelination.
Conclusion: NMDA-induced excitotoxic retinal injury causes mainly necrotic RGC somal death with Wallerian-like degeneration of the optic nerve. Since axonal degeneration associated with perikaryal excitotoxic injury is an active, regulated process, it may be amenable to therapeutic intervention.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

