T3 affects expression of collagen I and collagen cross-linking in bone cell cultures
- PMID: 20707983
- PMCID: PMC3025330
- DOI: 10.1016/j.bbrc.2010.08.022
T3 affects expression of collagen I and collagen cross-linking in bone cell cultures
Abstract
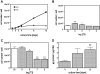
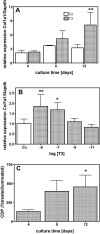
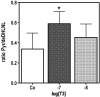
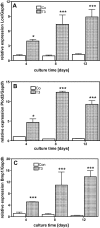
Thyroid hormones (T3,T4) have a broad range of effects on bone, however, its role in determining the quality of bone matrix is poorly understood. In-vitro, the immortalized mouse osteoblast-like cell line MC3T3-E1 forms a tissue like structure, consisting of several cell layers, whose formation is affected by T3 significantly. In this culture system, we investigated the effects of T3 on cell multiplication, collagen synthesis, expression of genes related to the collagen cross-linking process and on the formation of cross-links. T3 compared to controls modulated cell multiplication, up-regulated collagen synthesis time and dose dependently, and stimulated protein synthesis. T3 increased mRNA expressions of procollagen-lysine-1,2-oxoglutarate 5-dioxygenase 2 (Plod2) and of lysyloxidase (Lox), both genes involved in post-translational modification of collagen. Moreover, it stimulated mRNA expression of bone morphogenetic protein 1 (Bmp1), the processing enzyme of the lysyloxidase-precursor and of procollagen. An increase in the collagen cross-link-ratio Pyr/deDHLNL indicates, that T3 modulated cross-link maturation in the MC3T3-E1 culture system. These results demonstrate that T3 directly regulates collagen synthesis and collagen cross-linking by up-regulating gene expression of the specific cross-link related enzymes, and underlines the importance of a well-balanced concentration of thyroid hormones for maintenance of bone quality.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Murphy E., Williams G.R. The thyroid and the skeleton. Clin. Endocrinol. (Oxf.) 2004;61:285–298. - PubMed
-
- Allain T.J., McGregor A.M. Thyroid hormones and bone. J. Endocrinol. 1993;139:9–18. - PubMed
-
- Nilsson O., Marino R., De Luca F., Phillip M., Baron J. Endocrine regulation of the growth plate. Horm. Res. 2005;64:157–165. - PubMed
-
- Harvey C.B., O’Shea P.J., Scott A.J., Robson H., Siebler T., Shalet S.M., Samarut J., Chassande O., Williams G.R. Molecular mechanisms of thyroid hormone effects on bone growth and function. Mol. Genet. Metab. 2002;75:17–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

