Regulation of tumor angiogenesis by EZH2
- PMID: 20708159
- PMCID: PMC2923653
- DOI: 10.1016/j.ccr.2010.06.016
Regulation of tumor angiogenesis by EZH2
Abstract
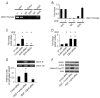
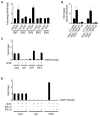
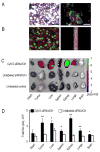
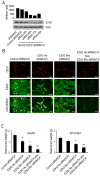
Although VEGF-targeted therapies are showing promise, new angiogenesis targets are needed to make additional gains. Here, we show that increased Zeste homolog 2 (EZH2) expression in either tumor cells or in tumor vasculature is predictive of poor clinical outcome. The increase in endothelial EZH2 is a direct result of VEGF stimulation by a paracrine circuit that promotes angiogenesis by methylating and silencing vasohibin1 (vash1). Ezh2 silencing in the tumor-associated endothelial cells inhibited angiogenesis mediated by reactivation of VASH1, and reduced ovarian cancer growth, which is further enhanced in combination with ezh2 silencing in tumor cells. Collectively, these data support the potential for targeting ezh2 as an important therapeutic approach.
2010 Elsevier Inc. All rights reserved.
Figures


References
-
- Ali-Fehmi R, Morris RT, Bandyopadhyay S, Che M, Schimp V, Malone JM, Jr, Munkarah AR. Expression of cyclooxygenase-2 in advanced stage ovarian serous carcinoma: correlation with tumor cell proliferation, apoptosis, angiogenesis, and survival. Am J Obstet Gynecol. 2005;192:819–825. - PubMed
-
- Burger RA, Sill MW, Monk BJ, Greer BE, Sorosky JI. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:5165–5171. - PubMed
-
- Burger RA, Brady MF, Bookman MA, Walker JL, Homesle HD, Fowler J, Monk BJ, Greer BE, Boente M, Liang SX. Phase lll trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): a Gynecologic Oncology Group Study. J Clin Oncol. 2010;28:7s.
-
- Cao R, Zhang Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr Opin Genet Dev. 2004;14:155–164. - PubMed
-
- Cavalli G, Paro R. Chromo-domain proteins: linking chromatin structure to epigenetic regulation. Curr Opin Cell Biol. 1998;10:354–360. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- HD050128/HD/NICHD NIH HHS/United States
- P50 CA098258/CA/NCI NIH HHS/United States
- P50 CA083639/CA/NCI NIH HHS/United States
- R01 CA128797/CA/NCI NIH HHS/United States
- CA 109298/CA/NCI NIH HHS/United States
- K12 HD050128/HD/NICHD NIH HHS/United States
- CA 110793/CA/NCI NIH HHS/United States
- T32 CA101642/CA/NCI NIH HHS/United States
- CA128797/CA/NCI NIH HHS/United States
- R01 CA109298/CA/NCI NIH HHS/United States
- RC2GM092599/GM/NIGMS NIH HHS/United States
- R01 CA110793/CA/NCI NIH HHS/United States
- RC2 GM092599/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

