Imaging of brain tumors: MR spectroscopy and metabolic imaging
- PMID: 20708548
- PMCID: PMC2927327
- DOI: 10.1016/j.nic.2010.04.003
Imaging of brain tumors: MR spectroscopy and metabolic imaging
Abstract
The utility of magnetic resonance spectroscopy (MRS) in diagnosis and evaluation of treatment response to human brain tumors has been widely documented. The role of MRS in tumor classification, tumors versus nonneoplastic lesions, prediction of survival, treatment planning, monitoring of therapy, and post-therapy evaluation is discussed. This article delineates the need for standardization and further study in order for MRS to become widely used as a routine clinical tool.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



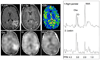
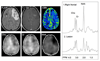

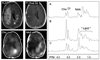

References
-
- Frahm J, Bruhn H, Gyngell ML, et al. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med. 1989;9(1):79–93. - PubMed
-
- Bruhn H, Frahm J, Gyngell ML, et al. Noninvasive differentiation of tumors with use of localized H-1 MR spectroscopy in vivo: initial experience in patients with cerebral tumors. Radiology. 1989;172(2):541–548. - PubMed
-
- Barker PB, Glickson JD, Bryan RN, et al. In vivo magnetic resonance spectroscopy of human brain tumors. Top Magn Reson Imaging. 1993;5(1):32–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

