Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling
- PMID: 20708588
- PMCID: PMC3291717
- DOI: 10.1016/j.devcel.2010.07.015
Focal adhesion kinase is required for intestinal regeneration and tumorigenesis downstream of Wnt/c-Myc signaling
Erratum in
-
Focal Adhesion Kinase Is Required for Intestinal Regeneration and Tumorigenesis Downstream of Wnt/c-Myc Signaling.Dev Cell. 2025 Jun 9;60(11):1649. doi: 10.1016/j.devcel.2025.05.001. Epub 2025 May 10. Dev Cell. 2025. PMID: 40349707 No abstract available.
Abstract
The intestinal epithelium has a remarkable capacity to regenerate after injury and DNA damage. Here, we show that the integrin effector protein Focal Adhesion Kinase (FAK) is dispensable for normal intestinal homeostasis and DNA damage signaling, but is essential for intestinal regeneration following DNA damage. Given Wnt/c-Myc signaling is activated following intestinal regeneration, we investigated the functional importance of FAK following deletion of the Apc tumor suppressor protein within the intestinal epithelium. Following Apc loss, FAK expression increased in a c-Myc-dependent manner. Codeletion of Apc and Fak strongly reduced proliferation normally induced following Apc loss, and this was associated with reduced levels of phospho-Akt and suppression of intestinal tumorigenesis in Apc heterozygous mice. Thus, FAK is required downstream of Wnt Signaling, for Akt/mTOR activation, intestinal regeneration, and tumorigenesis. Importantly, this work suggests that FAK inhibitors may suppress tumorigenesis in patients at high risk of developing colorectal cancer.
2010 Elsevier Inc. All rights reserved.
Figures
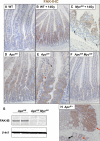

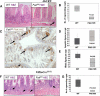

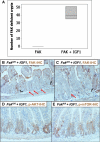

Comment in
-
Getting one's Fak straight.Dev Cell. 2010 Aug 17;19(2):185-6. doi: 10.1016/j.devcel.2010.07.023. Dev Cell. 2010. PMID: 20708578 Free PMC article.
References
-
- Agochiya M, Brunton VG, Owens DW, Parkinson EK, Paraskeva C, Keith WN, Frame MC. Increased dosage and amplification of the focal adhesion kinase gene in human cancer cells. Oncogene. 1999;18:5646–5653. - PubMed
-
- Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. - PubMed
-
- Athineos D, Sansom OJ. Myc heterozygosity attenuates the phenotypes of APC deficiency in the small intestine. Oncogene. 2010;29:2585–2590. - PubMed
-
- Bach SP, Renehan AG, Potten CS. Stem cells: the intestinal stem cell as a paradigm. Carcinogenesis. 2000;21:469–476. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

