Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels
- PMID: 20708594
- PMCID: PMC3540406
- DOI: 10.1016/j.devcel.2010.07.010
Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels
Abstract
During endochondral bone development, the first osteoblasts differentiate in the perichondrium surrounding avascular cartilaginous rudiments; the source of trabecular osteoblasts inside the later bone is, however, unknown. Here, we generated tamoxifen-inducible transgenic mice bred to Rosa26R-LacZ reporter mice to follow the fates of stage-selective subsets of osteoblast lineage cells. Pulse-chase studies showed that osterix-expressing osteoblast precursors, labeled in the perichondrium prior to vascular invasion of the cartilage, give rise to trabecular osteoblasts, osteocytes, and stromal cells inside the developing bone. Throughout the translocation, some precursors were found to intimately associate with invading blood vessels, in pericyte-like fashion. A similar coinvasion occurs during endochondral healing of bone fractures. In contrast, perichondrial mature osteoblasts did not exhibit perivascular localization and remained in the outer cortex of developing bones. These findings reveal the specific involvement of immature osteoblast precursors in the coupled vascular and osteogenic transformation essential to endochondral bone development and repair.
2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare no conflicts of interest.
Figures
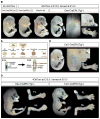
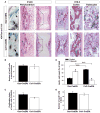

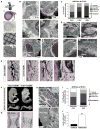

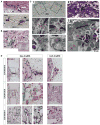

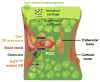
Comment in
-
On bone-forming cells and blood vessels in bone development.Cell Metab. 2010 Oct 6;12(4):314-316. doi: 10.1016/j.cmet.2010.09.009. Cell Metab. 2010. PMID: 20889123 Free PMC article.
References
-
- Bianco P, Cancedda FD, Riminucci M, Cancedda R. Bone formation via cartilage models: the “borderline” chondrocyte. Matrix Biol. 1998;17:185–192. - PubMed
-
- Colnot C, Lu C, Hu D, Helms JA. Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development. Dev Biol. 2004;269:55–69. - PubMed
-
- Colnot C, de la Fuente L, Huang S, Hu D, Lu C, St-Jacques B, Helms JA. Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development. Development. 2005;132:1057–1067. - PubMed
-
- Galotto M, Campanile G, Robino G, Cancedda FD, Bianco P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. J Bone Miner Res. 1994;9:1239–1249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

