αA-Crystallin associates with α6 integrin receptor complexes and regulates cellular signaling
- PMID: 20709056
- PMCID: PMC2962712
- DOI: 10.1016/j.exer.2010.08.006
αA-Crystallin associates with α6 integrin receptor complexes and regulates cellular signaling
Abstract
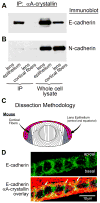
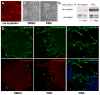
α-Crystallins are small heat-shock proteins important to lens transparency that provide the lens with its refractive properties. In their role as molecular chaperones, these crystallins also prevent protein aggregation, affect cytoskeletal remodeling, enhance resistance to cell stress, and provide lens cells with protection against apoptosis. While many of the functions assigned to αA-crystallin are attributable to its presence in the cytoplasm of lens cells, αA-crystallin also has been detected at the lens plasma membrane. However, how αA-crystallin becomes linked to the plasma membrane or what its functions are at this site has remained unknown. In this study, we examined the mechanisms by which αA-crystallin becomes associated with the lens membrane, focusing specifically on its interaction with membrane receptors, and the differentiation-specificity of these interactions. We also determined how the long-term absence of αA-crystallin alters receptor-linked signaling pathways. αA-crystallin association with membrane receptors was determined by co-immunoprecipitation analysis; its membrane localization was examined by confocal imaging; and the effect of αA-crystallin loss-of-function on the activation state of signaling molecules in pathways linked to membrane receptors was determined by immunoblot analysis. The results show that, in lens epithelial cells, plasma membrane αA-crystallin was primarily localized to apicolateral borders, reflecting the association of αA-crystallin with E-cadherin complexes. These studies also provide the first evidence that αA-crystallin maintained its association with the plasma membrane in lens cortical fiber cells, where it was localized to lateral interfaces, and further show that this association was mediated, in part, by αA-crystallin interaction with α6 integrin receptor complexes. We report that the absence of αA-crystallin led to constitutive activation of the stress kinases p38 and JNK, classical inducers of apoptotic cell death, and the loss of the phospho-Bad pro-survival signal, effects that were greatest in differentiating lens fiber cells. Concurrent with this, activation of FAK and ERK kinases was increased, demonstrating that these receptor-linked pathways also were dysregulated in the absence of αA-crystallin. These data link αA-crystallin plasma membrane association to its differentiation-state-specific interaction with E-cadherin and α6 integrin receptor complexes. The changes in cell signaling in αA-crystallin-null lenses suggest that dysregulation of receptor-linked cell-signaling pathways that accompany the failure of αA-crystallin to associate with membrane receptors may be responsible for the induction of apoptosis. The observed changes in lens cell signaling likely reflect long-term functional adaptations to the absence of the αA-crystallin chaperone/small heat-shock protein.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
alpha-Crystallin localizes to the leading edges of migrating lens epithelial cells.Exp Cell Res. 2005 May 15;306(1):203-15. doi: 10.1016/j.yexcr.2005.01.026. Epub 2005 Mar 17. Exp Cell Res. 2005. PMID: 15878345
-
Loss of the small heat shock protein αA-crystallin does not lead to detectable defects in early zebrafish lens development.Exp Eye Res. 2013 Nov;116:227-33. doi: 10.1016/j.exer.2013.09.007. Epub 2013 Sep 25. Exp Eye Res. 2013. PMID: 24076322 Free PMC article.
-
Loss of αBa-crystallin, but not αA-crystallin, increases age-related cataract in the zebrafish lens.Exp Eye Res. 2024 Jul;244:109918. doi: 10.1016/j.exer.2024.109918. Epub 2024 May 3. Exp Eye Res. 2024. PMID: 38705506
-
Effects of alpha-crystallin on lens cell function and cataract pathology.Curr Mol Med. 2009 Sep;9(7):887-92. doi: 10.2174/156652409789105598. Curr Mol Med. 2009. PMID: 19860667 Review.
-
The lens epithelium: focus on the expression and function of the alpha-crystallin chaperones.Int J Biochem Cell Biol. 2008;40(3):317-23. doi: 10.1016/j.biocel.2007.10.034. Epub 2007 Nov 13. Int J Biochem Cell Biol. 2008. PMID: 18093866 Free PMC article. Review.
Cited by
-
α6 integrin transactivates insulin-like growth factor receptor-1 (IGF-1R) to regulate caspase-3-mediated lens epithelial cell differentiation initiation.J Biol Chem. 2014 Feb 14;289(7):3842-55. doi: 10.1074/jbc.M113.515254. Epub 2013 Dec 31. J Biol Chem. 2014. PMID: 24381169 Free PMC article.
-
Novel roles for α-crystallins in retinal function and disease.Prog Retin Eye Res. 2012 Nov;31(6):576-604. doi: 10.1016/j.preteyeres.2012.06.001. Epub 2012 Jun 18. Prog Retin Eye Res. 2012. PMID: 22721717 Free PMC article. Review.
-
Mutation analysis of two families with inherited congenital cataracts.Mol Med Rep. 2015 Sep;12(3):3469-3475. doi: 10.3892/mmr.2015.3819. Epub 2015 May 22. Mol Med Rep. 2015. PMID: 26004348 Free PMC article.
-
α-Crystallin promotes rat axonal regeneration through regulation of RhoA/rock/cofilin/MLC signaling pathways.J Mol Neurosci. 2012 Jan;46(1):138-44. doi: 10.1007/s12031-011-9537-z. Epub 2011 May 17. J Mol Neurosci. 2012. PMID: 21584655
-
Creatine kinase/α-crystallin interaction functions in cataract development.Biochem Biophys Rep. 2020 Feb 29;22:100748. doi: 10.1016/j.bbrep.2020.100748. eCollection 2020 Jul. Biochem Biophys Rep. 2020. PMID: 32154391 Free PMC article.
References
-
- Ahmed M, Forsberg J, Bergsten P. Protein profiling of human pancreatic islets by two-dimensional gel electrophoresis and mass spectrometry. J Proteome Res. 2005;4:931–940. - PubMed
-
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. - PubMed
-
- Andley UP, Mathur S, Griest TA, Petrash JM. Cloning, expression, and chaperone-like activity of human alphαA-crystallin. J Biol Chem. 1996;271:31973–31980. - PubMed
-
- Andley UP, Patel HC, Xi JH. The R116C mutation in alpha A-crystallin diminishes its protective ability against stress-induced lens epithelial cell apoptosis. J Biol Chem. 2002;277:10178–10186. - PubMed
-
- Andley UP, Rhim JS, Chylack LT, Jr, Fleming TP. Propagation and immortalization of human lens epithelial cells in culture. Invest Ophthalmol Vis Sci. 1994;35:3094–3102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

