The effect of restraint stress on prepulse inhibition and on corticotropin-releasing factor (CRF) and CRF receptor gene expression in Wistar-Kyoto and Brown Norway rats
- PMID: 20709096
- PMCID: PMC2956775
- DOI: 10.1016/j.pbb.2010.08.003
The effect of restraint stress on prepulse inhibition and on corticotropin-releasing factor (CRF) and CRF receptor gene expression in Wistar-Kyoto and Brown Norway rats
Abstract
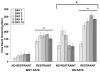
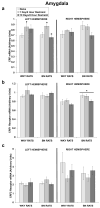
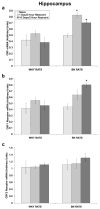
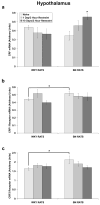
Stress plays a role in many psychiatric disorders that are characterized by deficits in prepulse inhibition (PPI), a form of sensorimotor gating. Corticotropin-releasing factor (CRF) is one of the most important neurotransmitters involved in behavioral components of the stress response, and central infusion of CRF decreases PPI in rodents. We recently demonstrated that restraint stress decreases PPI and attenuates the increase in PPI caused by repeated testing. To broaden our investigation into how restraint affects PPI, we subjected Wistar-Kyoto (WKY) and Brown Norway (BN) rats to 10 consecutive days of 2-hour restraint, or to brief handling, prior to assessing PPI. We next examined the effects of 1 or 10days of 2-hour restraint on plasma corticosterone levels in order to determine whether the endocrine response to stress parallels the behavioral effect of stress. Finally, we examined the effects of 1 or 10days of 2-hour restraint on CRF and CRF receptor gene expression in the amygdala, hippocampus, frontal cortex, and hypothalamus in order to determine whether a temporal pattern of gene expression parallels the change in the behavioral response to stress. The major findings of the present study are that 1) restraint stress attenuates the increase in PPI caused by repeated testing in both WKY and BN rats, and BN rats are more sensitive to the effects of restraint on PPI than WKY rats, 2) restraint-induced increases in corticosterone levels mirror the effect of restraint on PPI in WKY rats but not in BN rats, 3) laterality effects on gene expression were observed for the amygdala, whereby restraint increases CRF gene expression in the left, but not right, amygdala, and 4) some restraint-induced changes in CRF and CRF receptor gene expression precede changes in PPI while other changes coincide with altered PPI in a rat strain- and brain region-dependent manner.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures


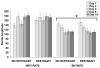


References
-
- Alsene KM, Carasso BS, Connors EE, Bakshi VP. Disruption of prepulse inhibition after stimulation of central but not peripheral alpha-1 adrenergic receptors. Neuropsychopharmacology. 2006;31:2150–61. - PubMed
-
- Baker KB, Kim JJ. Amygdalar lateralization in fear conditioning: evidence for greater involvement of the right amygdala. Behav Neurosci. 2004;118:15–23. - PubMed
-
- Balagura S, Harrell LE, Roy E. Effect of the light-dark cycle on neuroendocrine and behavioral responses to scheduled feeding. Physiol Behav. 1975;15:245–7. - PubMed
-
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. - PubMed
-
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

