Bifunctional fusion proteins of the human engineered antibody domain m36 with human soluble CD4 are potent inhibitors of diverse HIV-1 isolates
- PMID: 20709110
- PMCID: PMC2954370
- DOI: 10.1016/j.antiviral.2010.08.004
Bifunctional fusion proteins of the human engineered antibody domain m36 with human soluble CD4 are potent inhibitors of diverse HIV-1 isolates
Abstract
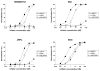
Currently used antiretroviral therapy is highly successful but there is still a need for new effective and safe prophylactics and therapeutics. We have previously identified and characterized a human engineered antibody domain (eAd), m36, which exhibits potent broadly neutralizing activity against HIV-1 by targeting a highly conserved CD4 binding-induced (CD4i) structure on the viral envelope glycoprotein (Env) gp120. m36 has very small size (∼15kDa) but is highly specific and is likely to be safe in long-term use thus representing a novel class of potentially promising HIV-1 inhibitors. Major problems with the development of m36 as a candidate therapeutic are possible short serum half life and lack of effector functions that could be important for effective protection in vivo. Fusion of m36 to human IgG1 Fc resulted in dramatically diminished neutralization potency most likely due to the sterically restricted nature of the m36 epitope that limits access of large molecules. To confer effector functions and simultaneously increase the potency, we first matured m36 by panning and screening a mutant library for mutants with increased binding to gp120. We next fused m36 and its mutants with the first two domains (soluble CD4, sCD4) of the human CD4 using a polypeptide linker. Our results showed that the selected m36 mutants and the sCD4 fusion proteins exhibited more potent antiviral activities than m36. The m36-sCD4 fusion proteins with human IgG1 Fc showed even higher potency likely due to their bivalency and increased avidity although with a greater increase in molecular size. Our data suggest that m36 derivatives are promising HIV-1 candidate therapeutics and tools to study highly conserved gp120 structures with implications for understanding mechanisms of entry and design of vaccine immunogens and small-molecule inhibitors.
Published by Elsevier B.V.
Figures
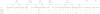



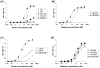
Similar articles
-
Human domain antibodies to conserved sterically restricted regions on gp120 as exceptionally potent cross-reactive HIV-1 neutralizers.Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):17121-6. doi: 10.1073/pnas.0805297105. Epub 2008 Oct 28. Proc Natl Acad Sci U S A. 2008. PMID: 18957538 Free PMC article.
-
Identification and characterization of a new cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody.J Virol. 2004 Sep;78(17):9233-42. doi: 10.1128/JVI.78.17.9233-9242.2004. J Virol. 2004. PMID: 15308718 Free PMC article.
-
Germlining of the HIV-1 broadly neutralizing antibody domain m36.Antiviral Res. 2015 Apr;116:62-6. doi: 10.1016/j.antiviral.2015.02.001. Epub 2015 Feb 9. Antiviral Res. 2015. PMID: 25676867 Free PMC article.
-
Human monoclonal antibodies and engineered antibody domains as HIV-1 entry inhibitors.Curr Opin HIV AIDS. 2009 Mar;4(2):112-7. doi: 10.1097/COH.0b013e328322f95e. Curr Opin HIV AIDS. 2009. PMID: 19339949 Free PMC article. Review.
-
Janusin: new molecular design for bispecific reagents.Int J Cancer Suppl. 1992;7:51-2. Int J Cancer Suppl. 1992. PMID: 1428404 Review.
Cited by
-
Bispecific Anti-HIV Immunoadhesins That Bind Gp120 and Gp41 Have Broad and Potent HIV-Neutralizing Activity.Vaccines (Basel). 2021 Jul 12;9(7):774. doi: 10.3390/vaccines9070774. Vaccines (Basel). 2021. PMID: 34358190 Free PMC article.
-
Bifunctional CD4-DC-SIGN fusion proteins demonstrate enhanced avidity to gp120 and inhibit HIV-1 infection and dissemination.Antimicrob Agents Chemother. 2012 Sep;56(9):4640-9. doi: 10.1128/AAC.00623-12. Epub 2012 Jun 11. Antimicrob Agents Chemother. 2012. PMID: 22687513 Free PMC article.
-
Monoclonal antibody-based candidate therapeutics against HIV type 1.AIDS Res Hum Retroviruses. 2012 May;28(5):425-34. doi: 10.1089/AID.2011.0226. Epub 2011 Sep 23. AIDS Res Hum Retroviruses. 2012. PMID: 21827278 Free PMC article. Review.
-
Candidate antibody-based therapeutics against HIV-1.BioDrugs. 2012 Jun 1;26(3):143-62. doi: 10.2165/11631400-000000000-00000. BioDrugs. 2012. PMID: 22462520 Free PMC article. Review.
-
HIV Entry and Its Inhibition by Bifunctional Antiviral Proteins.Mol Ther Nucleic Acids. 2018 Dec 7;13:347-364. doi: 10.1016/j.omtn.2018.09.003. Epub 2018 Sep 11. Mol Ther Nucleic Acids. 2018. PMID: 30340139 Free PMC article. Review.
References
-
- Choe H, Li W, Wright PL, Vasilieva N, Venturi M, Huang CC, Grundner C, Dorfman T, Zwick MB, Wang L, Rosenberg ES, Kwong PD, Burton DR, Robinson JE, Sodroski JG, Farzan M. Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell. 2003;114:161–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

