Characterization of glycidyl methacrylate - crosslinked hyaluronan hydrogel scaffolds incorporating elastogenic hyaluronan oligomers
- PMID: 20709199
- PMCID: PMC4024838
- DOI: 10.1016/j.actbio.2010.08.006
Characterization of glycidyl methacrylate - crosslinked hyaluronan hydrogel scaffolds incorporating elastogenic hyaluronan oligomers
Abstract
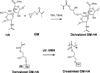
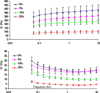
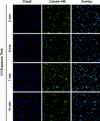
Prior studies on two-dimensional cell cultures suggest that hyaluronic acid (HA) stimulates cell-mediated regeneration of extracellular matrix structures, specifically those containing elastin, though such biologic effects are dependent on HA fragment size. Towards being able to regenerate three-dimensional (3-D) elastic tissue constructs, the present paper studies photo-crosslinked hydrogels containing glycidyl methacrylate (GM)-derivatized bio-inert high molecular weight (HMW) HA (1 × 10(6)Da) and a bioactive HA oligomer mixture (HA-o: MW ∼0.75 kDa). The mechanical (rheology, degradation) and physical (apparent crosslinking density, swelling ratio) properties of the gels varied as a function of incorporated HA oligomer content; however, overall, the mechanics of these hydrogels were too weak for vascular applications as stand-alone materials. Upon in vivo subcutaneous implantation, only a few inflammatory cells were evident around GM-HA gels, however their number increased as HA-o content within the gels increased, and the collagen I distribution was uniform. Smooth muscle cells (SMC) were encapsulated into GM hydrogels, and calcein acetoxymethyl detection revealed that the cells were able to endure twofold the level of UV exposure used to crosslink the gels. After 21 days of culture, SMC elastin production, measured by immunofluorescence quantification, showed HA-o to increase cellular deposition of elastic matrix twofold relative to HA-o-free GM-HA gels. These results demonstrate that cell response to HA/HA-o is not altered by their methacrylation and photo-crosslinking into a hydrogel, and that HA-o incorporation into cell-encapsulating hydrogel scaffolds can be useful for enhancing their production of elastic matrix structures in a 3-D space, important for regenerating elastic tissues.
Copyright © 2010. Published by Elsevier Ltd.
Figures








Similar articles
-
Elastogenic effects of exogenous hyaluronan oligosaccharides on vascular smooth muscle cells.Biomaterials. 2006 Nov;27(33):5698-707. doi: 10.1016/j.biomaterials.2006.07.020. Epub 2006 Aug 8. Biomaterials. 2006. PMID: 16899292
-
The impact of hyaluronic acid oligomer content on physical, mechanical, and biologic properties of divinyl sulfone-crosslinked hyaluronic acid hydrogels.J Biomed Mater Res A. 2010 Aug;94(2):355-70. doi: 10.1002/jbm.a.32704. J Biomed Mater Res A. 2010. PMID: 20186732 Free PMC article.
-
Benefits of concurrent delivery of hyaluronan and IGF-1 cues to regeneration of crosslinked elastin matrices by adult rat vascular cells.J Tissue Eng Regen Med. 2008 Mar-Apr;2(2-3):106-16. doi: 10.1002/term.70. J Tissue Eng Regen Med. 2008. PMID: 18338830
-
Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds.Acta Biomater. 2014 Apr;10(4):1571-1580. doi: 10.1016/j.actbio.2013.07.025. Epub 2013 Jul 27. Acta Biomater. 2014. PMID: 23899481 Free PMC article. Review.
-
Hyaluronan-Based Hydrogel Scaffolds for Limbal Stem Cell Transplantation: A Review.Cells. 2019 Mar 14;8(3):245. doi: 10.3390/cells8030245. Cells. 2019. PMID: 30875861 Free PMC article. Review.
Cited by
-
3D Freeform Printing of Nanocomposite Hydrogels through in situ Precipitation in Reactive Viscous Fluid.Int J Bioprint. 2020 Apr 2;6(2):258. doi: 10.18063/ijb.v6i2.258.. eCollection 2020. Int J Bioprint. 2020. PMID: 32782988 Free PMC article.
-
Advances in biomimetic regeneration of elastic matrix structures.Drug Deliv Transl Res. 2012 Oct;2(5):323-50. doi: 10.1007/s13346-012-0070-6. Drug Deliv Transl Res. 2012. PMID: 23355960 Free PMC article.
-
Collagen- and hyaluronic acid-based hydrogels and their biomedical applications.Mater Sci Eng R Rep. 2021 Oct;146:100641. doi: 10.1016/j.mser.2021.100641. Epub 2021 Jul 30. Mater Sci Eng R Rep. 2021. PMID: 34483486 Free PMC article.
-
Ex vivo enhancement of CD8+ T cell activity using functionalized hydrogel encapsulating tonsil-derived lymphatic endothelial cells.Theranostics. 2025 Jan 1;15(3):850-874. doi: 10.7150/thno.100079. eCollection 2025. Theranostics. 2025. PMID: 39776798 Free PMC article.
-
Photo-active collagen systems with controlled triple helix architecture.J Mater Chem B. 2013 Aug 14;1(30):3705-3715. doi: 10.1039/C3TB20720J. J Mater Chem B. 2013. PMID: 27398214 Free PMC article.
References
-
- Tan SW, Johns MR, Greenfield PF. Hyaluronic acid—a versatile biopolymer. Aust J Biotechnol. 1990;4:38–43. - PubMed
-
- Chen WY, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. - PubMed
-
- LJaL Lapcik, Lapcik L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: preparation, structure, properties, and applications. Chem Rev. 1998;98:2663–2684. - PubMed
-
- Shu XZ, Ghosh K, Liu Y, Palumbo FS, Luo Y, Clark RA, et al. Attachment and spreading of fibroblasts on an RGD peptide-modified injectable hyaluronan hydrogel. J Biomed Mater Res A. 2004;68:365–375. - PubMed
-
- Segura T, Anderson BC, Chung PH, Webber RE, Shull KR, Shea LD. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26:359–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

