Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin
- PMID: 20709295
- PMCID: PMC2929649
- DOI: 10.1016/j.chom.2010.07.007
Direct complement restriction of flavivirus infection requires glycan recognition by mannose-binding lectin
Abstract
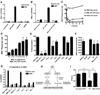
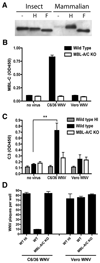
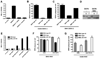
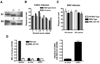
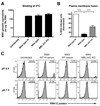
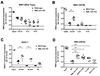
An intact complement system is crucial for limiting West Nile virus (WNV) dissemination. Herein, we define how complement directly restricts flavivirus infection in an antibody-independent fashion. Mannose-binding lectin (MBL) recognized N-linked glycans on the structural proteins of WNV and Dengue virus (DENV), resulting in neutralization through a C3- and C4-dependent mechanism that utilized both the canonical and bypass lectin activation pathways. For WNV, neutralization occurred with virus produced in insect cells, whereas for DENV, neutralization of insect and mammalian cell-derived virus was observed. Mechanism of action studies suggested that the MBL-dependent neutralization occurred, in part, by blocking viral fusion. Experiments in mice showed an MBL-dependent accelerated intravascular clearance of DENV or a WNV mutant with two N-linked glycans on its E protein, but not with wild-type WNV. Our studies show that MBL recognizes terminal mannose-containing carbohydrates on flaviviruses, resulting in neutralization and efficient clearance in vivo.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
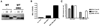
Similar articles
-
Complement-mediated neutralization of dengue virus requires mannose-binding lectin.mBio. 2011 Dec 13;2(6):e00276-11. doi: 10.1128/mBio.00276-11. Print 2011. mBio. 2011. PMID: 22167226 Free PMC article.
-
Dengue and the Lectin Pathway of the Complement System.Viruses. 2021 Jun 24;13(7):1219. doi: 10.3390/v13071219. Viruses. 2021. PMID: 34202570 Free PMC article. Review.
-
A plant-produced vaccine protects mice against lethal West Nile virus infection without enhancing Zika or dengue virus infectivity.Vaccine. 2018 Mar 27;36(14):1846-1852. doi: 10.1016/j.vaccine.2018.02.073. Epub 2018 Feb 26. Vaccine. 2018. PMID: 29490880 Free PMC article.
-
Secreted NS1 Protects Dengue Virus from Mannose-Binding Lectin-Mediated Neutralization.J Immunol. 2016 Nov 15;197(10):4053-4065. doi: 10.4049/jimmunol.1600323. Epub 2016 Oct 19. J Immunol. 2016. PMID: 27798151 Free PMC article.
-
Flavivirus cell entry and membrane fusion.Viruses. 2011 Feb;3(2):160-171. doi: 10.3390/v3020160. Epub 2011 Feb 22. Viruses. 2011. PMID: 22049308 Free PMC article. Review.
Cited by
-
C1q binding to dengue virus decreases levels of infection and inflammatory molecules transcription in THP-1 cells.Virus Res. 2014 Jan 22;179:231-4. doi: 10.1016/j.virusres.2013.11.007. Epub 2013 Nov 15. Virus Res. 2014. PMID: 24246304 Free PMC article.
-
Antibody Fc characteristics and effector functions correlate with protection from symptomatic dengue virus type 3 infection.Sci Transl Med. 2022 Jun 29;14(651):eabm3151. doi: 10.1126/scitranslmed.abm3151. Epub 2022 Jun 29. Sci Transl Med. 2022. PMID: 35767652 Free PMC article.
-
Human lectins and their roles in viral infections.Molecules. 2015 Jan 29;20(2):2229-71. doi: 10.3390/molecules20022229. Molecules. 2015. PMID: 25642836 Free PMC article. Review.
-
Transmission-blocking antibodies against mosquito C-type lectins for dengue prevention.PLoS Pathog. 2014 Feb 13;10(2):e1003931. doi: 10.1371/journal.ppat.1003931. eCollection 2014 Feb. PLoS Pathog. 2014. PMID: 24550728 Free PMC article.
-
A Factor I-Like Activity Associated with Chikungunya Virus Contributes to Its Resistance to the Human Complement System.J Virol. 2020 Mar 17;94(7):e02062-19. doi: 10.1128/JVI.02062-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941783 Free PMC article.
References
-
- Acioli-Santos B, Segat L, Dhalia R, Brito CA, Braga-Neto UM, Marques ET, Crovella S. MBL2 gene polymorphisms protect against development of thrombocytopenia associated with severe dengue phenotype. Hum Immunol. 2008;69:122–128. - PubMed
-
- Bathum L, Hansen H, Teisner B, Koch C, Garred P, Rasmussen K, Wang P. Association between combined properdin and mannose-binding lectin deficiency and infection with Neisseria meningitidis. Mol Immunol. 2006;43:473–479. - PubMed
-
- Bokisch VA, Top FH, Jr, Russell PK, Dixon FJ, Muller-Eberhard HJ. The potential pathogenic role of complement in dengue hemorrhagic shock syndrome. N Engl J Med. 1973;289:996–1000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

