Alpha-lipoic acid inhibits thrombin-induced fetal membrane weakening in vitro
- PMID: 20709392
- PMCID: PMC2945435
- DOI: 10.1016/j.placenta.2010.07.012
Alpha-lipoic acid inhibits thrombin-induced fetal membrane weakening in vitro
Abstract
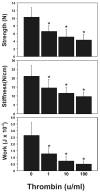
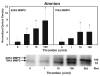
Cytokine-mediated inflammation and abruption-induced thrombin generation are separately implicated in matrix metalloproteinase (MMP)-mediated weakening of fetal membranes (FM) leading to preterm premature rupture of the fetal membranes (PPROM). At term, FM of both labored vaginal and unlabored Cesarean deliveries exhibit a weak zone overlying the cervix exhibiting ECM remodeling characterized by increased MMP9 protein and activity. We have reproduced these biochemical changes as well as FM weakening in vitro using tumor necrosis factor-alpha (TNF) and interleukin (IL)-1β, inflammatory cytokines implicated in PPROM. Additionally, we have reported that the antioxidant and NFκB inhibitor alpha-lipoic Acid (LA) blocks these TNF-induced effects. We now present the first direct evidence that thrombin also can induce FM weakening in vitro, and LA treatment inhibits this thrombin-induced-weakening. Full thickness FM fragments from unlabored Cesarean deliveries were incubated with increasing doses of thrombin (0-100 u/ml) for 48 h. Fragments were then strength tested (breaking force and work to rupture) using our published methodology. MMP3 and 9 levels in tissue extracts were determined by Western blot and densitometry. To determine the effect of LA, FM fragments were incubated with control medium or 10 u/ml thrombin, with or without 0.25 mM LA. Strength testing and MMP induction were determined. Thrombin induced a dose-dependent decrease in FM strength (42% baseline rupture force and 45% work to rupture) coupled with a dose-dependent increase in MMP3 and 9 expression (all p < 0.001). Treatment of FM with 0.25 mM LA completely inhibited thrombin-induced FM weakening and MMP expression (all p < 0.001). Thrombin treatment of cultured FM induces mechanical weakening and increased MMP3 and 9. Treatment of FM with LA inhibits these thrombin-induced effects. We speculate LA may prove clinically useful in prevention of PPROM associated with abruption.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures






Similar articles
-
The effects of thrombin and cytokines upon the biomechanics and remodeling of isolated amnion membrane, in vitro.Placenta. 2011 Mar;32(3):206-13. doi: 10.1016/j.placenta.2011.01.006. Placenta. 2011. PMID: 21300402 Free PMC article.
-
In an in-vitro model using human fetal membranes, α-lipoic acid inhibits inflammation induced fetal membrane weakening.Placenta. 2018 Aug;68:9-14. doi: 10.1016/j.placenta.2018.06.305. Epub 2018 Jun 19. Placenta. 2018. PMID: 30055672
-
Alpha-lipoic acid inhibits tumor necrosis factor-induced remodeling and weakening of human fetal membranes.Biol Reprod. 2009 Apr;80(4):781-7. doi: 10.1095/biolreprod.108.073205. Epub 2008 Dec 23. Biol Reprod. 2009. PMID: 19109223 Free PMC article.
-
The physiology of fetal membrane weakening and rupture: Insights gained from the determination of physical properties revisited.Placenta. 2016 Jun;42:59-73. doi: 10.1016/j.placenta.2016.03.015. Epub 2016 Apr 1. Placenta. 2016. PMID: 27238715 Review.
-
Mechanism of Human Fetal Membrane Biomechanical Weakening, Rupture and Potential Targets for Therapeutic Intervention.Obstet Gynecol Clin North Am. 2020 Dec;47(4):523-544. doi: 10.1016/j.ogc.2020.08.010. Obstet Gynecol Clin North Am. 2020. PMID: 33121643 Review.
Cited by
-
Progesterone receptor membrane component 1 as the mediator of the inhibitory effect of progestins on cytokine-induced matrix metalloproteinase 9 activity in vitro.Reprod Sci. 2014 Feb;21(2):260-8. doi: 10.1177/1933719113493514. Epub 2013 Jun 27. Reprod Sci. 2014. PMID: 23813454 Free PMC article.
-
Extracellular matrix dynamics and fetal membrane rupture.Reprod Sci. 2013 Feb;20(2):140-53. doi: 10.1177/1933719111424454. Epub 2012 Jan 19. Reprod Sci. 2013. PMID: 22267536 Free PMC article. Review.
-
Single Nucleotide Polymorphisms from CSF2, FLT1, TFPI and TLR9 Genes Are Associated with Prelabor Rupture of Membranes.Genes (Basel). 2021 Oct 28;12(11):1725. doi: 10.3390/genes12111725. Genes (Basel). 2021. PMID: 34828331 Free PMC article.
-
Benefits of α-lipoic acid in high-risk pregnancies (Review).Exp Ther Med. 2021 Nov;22(5):1232. doi: 10.3892/etm.2021.10666. Epub 2021 Aug 31. Exp Ther Med. 2021. PMID: 34539828 Free PMC article. Review.
-
Preterm prelabor rupture of the membranes: A disease of the fetal membranes.Semin Perinatol. 2017 Nov;41(7):409-419. doi: 10.1053/j.semperi.2017.07.012. Epub 2017 Aug 12. Semin Perinatol. 2017. PMID: 28807394 Free PMC article. Review.
References
-
- Mercer BM. Premature Rupture of the membranes. An expert’s view. Obstet Gynecol. 2003;101:178–193. - PubMed
-
- Menon R. Spontaneous preterm birth, a clinical dilemma: etiologic, pathophysiologic and genetic heterogeneities and racial disparity. Acta Obstet Gynecol Scand. 2008;87:590–600. - PubMed
-
- Parry S, Strauss JF. Premature rupture of the fetal membranes. N Engl J Med. 1998;338:663–670. - PubMed
-
- Menon R, Fortunato SJ. The role of matrix degrading enzymes and apoptosis in rupture of membranes. J Soc Gynecol Investig. 2004;11:427–437. - PubMed
-
- Moore RM, Mansour JM, Redline RW, Mercer BM, Moore JJ. The physiology of fetal membrane rupture: insight gained from the determination of physical properties. Placenta. 2006;27:1037–1051. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

