Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin
- PMID: 20709730
- PMCID: PMC3006274
- DOI: 10.1152/ajplung.00153.2010
Moderate postnatal hyperoxia accelerates lung growth and attenuates pulmonary hypertension in infant rats after exposure to intra-amniotic endotoxin
Abstract
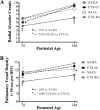
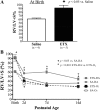
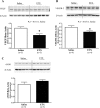
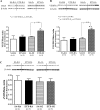
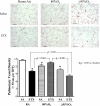
To determine the separate and interactive effects of fetal inflammation and neonatal hyperoxia on the developing lung, we hypothesized that: 1) antenatal endotoxin (ETX) causes sustained abnormalities of infant lung structure; and 2) postnatal hyperoxia augments the adverse effects of antenatal ETX on infant lung growth. Escherichia coli ETX or saline (SA) was injected into amniotic sacs in pregnant Sprague-Dawley rats at 20 days of gestation. Pups were delivered 2 days later and raised in room air (RA) or moderate hyperoxia (O₂, 80% O₂ at Denver's altitude, ∼65% O₂ at sea level) from birth through 14 days of age. Heart and lung tissues were harvested for measurements. Intra-amniotic ETX caused right ventricular hypertrophy (RVH) and decreased lung vascular endothelial growth factor (VEGF) and VEGF receptor-2 (VEGFR-2) protein contents at birth. In ETX-exposed rats (ETX-RA), alveolarization and vessel density were decreased, pulmonary vascular wall thickness percentage was increased, and RVH was persistent throughout the study period compared with controls (SA-RA). After antenatal ETX, moderate hyperoxia increased lung VEGF and VEGFR-2 protein contents in ETX-O₂ rats and improved their alveolar and vascular structure and RVH compared with ETX-RA rats. In contrast, severe hyperoxia (≥95% O₂ at Denver's altitude) further reduced lung vessel density after intra-amniotic ETX exposure. We conclude that intra-amniotic ETX induces fetal pulmonary hypertension and causes persistent abnormalities of lung structure with sustained pulmonary hypertension in infant rats. Moreover, moderate postnatal hyperoxia after antenatal ETX restores lung growth and prevents pulmonary hypertension during infancy.
Figures







References
-
- Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC. The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-wk preterm newborn infants. Am J Obstet Gynecol 195: 803–808, 2006 - PubMed
-
- Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol 291: L119–L127, 2006 - PubMed
-
- Bancalari E, Claure N. Evolving clinical features of bronchopulmonary dysplasia. In: Bronchopulmonary Dysplasia. New York: Informa Health Care, 2010, p.208–222
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

