The Saccharomyces cerevisiae anaphase-promoting complex interacts with multiple histone-modifying enzymes to regulate cell cycle progression
- PMID: 20709786
- PMCID: PMC2950418
- DOI: 10.1128/EC.00097-10
The Saccharomyces cerevisiae anaphase-promoting complex interacts with multiple histone-modifying enzymes to regulate cell cycle progression
Abstract
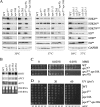
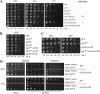
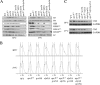
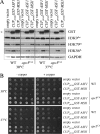
The anaphase-promoting complex (APC), a large evolutionarily conserved ubiquitin ligase complex, regulates cell cycle progression through mitosis and G(1). Here, we present data suggesting that APC-dependent cell cycle progression relies on a specific set of posttranslational histone-modifying enzymes. Multiple APC subunit mutants were impaired in total and modified histone H3 protein content. Acetylated H3K56 (H3K56(Ac)) levels were as reduced as those of total H3, indicating that loading histones with H3K56(Ac) is unaffected in APC mutants. However, under restrictive conditions, H3K9(Ac) and dimethylated H3K79 (H3K79(me2)) levels were more greatly reduced than those of total H3. In a screen for histone acetyltransferase (HAT) and histone deacetylase (HDAC) mutants that genetically interact with the apc5(CA) (chromatin assembly) mutant, we found that deletion of GCN5 or ELP3 severely hampered apc5(CA) temperature-sensitive (ts) growth. Further analyses showed that (i) the elp3Δ gcn5Δ double mutant ts defect was epistatic to that observed in apc5(CA) cells; (ii) gcn5Δ and elp3Δ mutants accumulate in mitosis; and (iii) turnover of the APC substrate Clb2 is not impaired in elp3Δ gcn5Δ cells. Increased expression of ELP3 and GCN5, as well as genes encoding the HAT Rtt109 and the chromatin assembly factors Msi1 and Asf1, suppressed apc5(CA) defects, while increased APC5 expression partially suppressed elp3Δ gcn5Δ growth defects. Finally, we demonstrate that Gcn5 is unstable during G(1) and following G(1) arrest and is stabilized in APC mutants. We present our working model in which Elp3/Gcn5 and the APC work together to facilitate passage through mitosis and G(1). To progress into S, we propose that at least Gcn5 must then be targeted for degradation in an APC-dependent fashion.
Figures



References
-
- Ausubel F. M., Brent R., Kingston R. E., Moore D. D., Seidman J. G., Smith J. A., Struhl K. 1995. Current protocols in molecular biology. Wiley, New York, NY
-
- Baker D. J., Jeganathan K. B., Cameron J. D., Thompson M., Juneja S., Kopecka A., Kumar R., Jenkins R. B., de Groen P. C., Roche P., van Deursen J. M. 2004. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat. Genet. 36:744–749 - PubMed
-
- Bellanger S., Blachon S., Mechali F., Bonne-Andrea C., Thierry F. 2005. High-risk but not low-risk HPV E2 proteins bind to the APC activators Cdh1 and Cdc20 and cause genomic instability. Cell Cycle 4:1608–1615 - PubMed
-
- Binné U. K., Classon M. K., Dick F. A., Wei W., Rape M., Kaelin W. G., Jr., Näär A. M., Dyson N. J. 2007. Retinoblastoma protein and anaphase-promoting complex physically interact and functionally cooperate during cell-cycle exit. Nat. Cell Biol. 9:225–232 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

