Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast
- PMID: 20709789
- PMCID: PMC2950420
- DOI: 10.1128/EC.00124-10
Phosphatidylinositol 3-phosphate, an essential lipid in Plasmodium, localizes to the food vacuole membrane and the apicoplast
Abstract
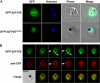
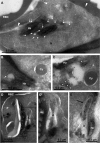
Phosphoinositides are important regulators of diverse cellular functions, and phosphatidylinositol 3-monophosphate (PI3P) is a key element in vesicular trafficking processes. During its intraerythrocytic development, the malaria parasite Plasmodium falciparum establishes a sophisticated but poorly characterized protein and lipid trafficking system. Here we established the detailed phosphoinositide profile of P. falciparum-infected erythrocytes and found abundant amounts of PI3P, while phosphatidylinositol 3,5-bisphosphate was not detected. PI3P production was parasite dependent, sensitive to a phosphatidylinositol-3-kinase (PI3-kinase) inhibitor, and predominant in late parasite stages. The Plasmodium genome encodes a class III PI3-kinase of unusual size, containing large insertions and several repetitive sequence motifs. The gene could not be deleted in Plasmodium berghei, and in vitro growth of P. falciparum was sensitive to a PI3-kinase inhibitor, indicating that PI3-kinase is essential in Plasmodium blood stages. For intraparasitic PI3P localization, transgenic P. falciparum that expressed a PI3P-specific fluorescent probe was generated. Fluorescence was associated mainly with the membrane of the food vacuole and with the apicoplast, a four-membrane bounded plastid-like organelle derived from an ancestral secondary endosymbiosis event. Electron microscopy analysis confirmed these findings and revealed, in addition, the presence of PI3P-positive single-membrane vesicles. We hypothesize that these vesicles might be involved in transport processes, likely of proteins and lipids, toward the essential and peculiar parasite compartment, which is the apicoplast. The fact that PI3P metabolism and function in Plasmodium appear to be substantially different from those in its human host could offer new possibilities for antimalarial chemotherapy.
Figures





Similar articles
-
Plasmodium falciparum Atg18 localizes to the food vacuole via interaction with the multi-drug resistance protein 1 and phosphatidylinositol 3-phosphate.Biochem J. 2021 May 14;478(9):1705-1732. doi: 10.1042/BCJ20210001. Biochem J. 2021. PMID: 33843972
-
PI3-kinase has multiple functions in asexual blood stages of Plasmodium falciparum.Sci Rep. 2025 May 14;15(1):16762. doi: 10.1038/s41598-025-01397-1. Sci Rep. 2025. PMID: 40369090 Free PMC article.
-
Traffic to the malaria parasite food vacuole: a novel pathway involving a phosphatidylinositol 3-phosphate-binding protein.J Biol Chem. 2007 Apr 13;282(15):11499-508. doi: 10.1074/jbc.M610974200. Epub 2007 Feb 8. J Biol Chem. 2007. PMID: 17289673
-
Vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes.Int J Parasitol. 2001 Oct;31(12):1381-91. doi: 10.1016/s0020-7519(01)00256-9. Int J Parasitol. 2001. PMID: 11566305 Review.
-
PIK-ing New Malaria Chemotherapy.Trends Parasitol. 2018 Nov;34(11):925-927. doi: 10.1016/j.pt.2018.06.003. Epub 2018 Jun 19. Trends Parasitol. 2018. PMID: 29934102 Review.
Cited by
-
Phosphatidylinositol 3-phosphate and Hsp70 protect Plasmodium falciparum from heat-induced cell death.Elife. 2020 Sep 25;9:e56773. doi: 10.7554/eLife.56773. Elife. 2020. PMID: 32975513 Free PMC article.
-
Plasmodium falciparum resistance to ACTs: Emergence, mechanisms, and outlook.Int J Parasitol Drugs Drug Resist. 2021 Aug;16:102-118. doi: 10.1016/j.ijpddr.2021.05.007. Epub 2021 May 26. Int J Parasitol Drugs Drug Resist. 2021. PMID: 34090067 Free PMC article.
-
PMRT1, a Plasmodium-Specific Parasite Plasma Membrane Transporter, Is Essential for Asexual and Sexual Blood Stage Development.mBio. 2022 Apr 26;13(2):e0062322. doi: 10.1128/mbio.00623-22. Epub 2022 Apr 11. mBio. 2022. PMID: 35404116 Free PMC article.
-
Lipid kinases are essential for apicoplast homeostasis in Toxoplasma gondii.Cell Microbiol. 2015 Apr;17(4):559-78. doi: 10.1111/cmi.12383. Epub 2014 Nov 22. Cell Microbiol. 2015. PMID: 25329540 Free PMC article.
-
Artemisinin susceptibility in the malaria parasite Plasmodium falciparum: propellers, adaptor proteins and the need for cellular healing.FEMS Microbiol Rev. 2021 May 5;45(3):fuaa056. doi: 10.1093/femsre/fuaa056. FEMS Microbiol Rev. 2021. PMID: 33095255 Free PMC article. Review.
References
-
- Allan D. 1982. Inositol lipids and membrane function in erythrocytes. Cell Calcium 3:451–465 - PubMed
-
- Auger K. R., Carpenter C. L., Cantley L. C., Varticovski L. 1989. Phosphatidylinositol 3-kinase and its novel product, phosphatidylinositol 3-phosphate, are present in Saccharomyces cerevisiae. J. Biol. Chem. 264:20181–20184 - PubMed
-
- Ayong L., Pagnotti G., Tobon A. B., Chakrabarti D. 2007. Identification of Plasmodium falciparum family of SNAREs. Mol. Biochem. Parasitol. 152:113–122 - PubMed
-
- Backer J. M. 2008. The regulation and function of class III PI3Ks: novel roles for Vps34. Biochem. J. 410:1–17 - PubMed
-
- Billker O., Dechamps S., Tewari R., Wenig G., Franke-Fayard B., Brinkmann V. 2004. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 117:503–514 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

