The ROCO kinase QkgA is necessary for proliferation inhibition by autocrine signals in Dictyostelium discoideum
- PMID: 20709790
- PMCID: PMC2950431
- DOI: 10.1128/EC.00121-10
The ROCO kinase QkgA is necessary for proliferation inhibition by autocrine signals in Dictyostelium discoideum
Abstract
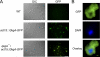
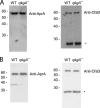
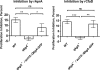
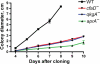
AprA and CfaD are secreted proteins that function as autocrine signals to inhibit cell proliferation in Dictyostelium discoideum. Cells lacking AprA or CfaD proliferate rapidly, and adding AprA or CfaD to cells slows proliferation. Cells lacking the ROCO kinase QkgA proliferate rapidly, with a doubling time 83% of that of the wild type, and overexpression of a QkgA-green fluorescent protein (GFP) fusion protein slows cell proliferation. We found that qkgA(-) cells accumulate normal levels of extracellular AprA and CfaD. Exogenous AprA or CfaD does not slow the proliferation of cells lacking qkgA, and expression of QkgA-GFP in qkgA(-) cells rescues this insensitivity. Like cells lacking AprA or CfaD, cells lacking QkgA tend to be multinucleate, accumulate nuclei rapidly, and show a mass and protein accumulation per nucleus like those of the wild type, suggesting that QkgA negatively regulates proliferation but not growth. Despite their rapid proliferation, cells lacking AprA, CfaD, or QkgA expand as a colony on bacteria less rapidly than the wild type. Unlike AprA and CfaD, QkgA does not affect spore viability following multicellular development. Together, these results indicate that QkgA is necessary for proliferation inhibition by AprA and CfaD, that QkgA mediates some but not all of the effects of AprA and CfaD, and that QkgA may function downstream of these proteins in a signal transduction pathway regulating proliferation.
Figures


Similar articles
-
Using Dictyostelium to Develop Therapeutics for Acute Respiratory Distress Syndrome.Front Cell Dev Biol. 2021 Jul 19;9:710005. doi: 10.3389/fcell.2021.710005. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34350188 Free PMC article. Review.
-
The putative bZIP transcription factor BzpN slows proliferation and functions in the regulation of cell density by autocrine signals in Dictyostelium.PLoS One. 2011;6(7):e21765. doi: 10.1371/journal.pone.0021765. Epub 2011 Jul 7. PLoS One. 2011. PMID: 21760904 Free PMC article.
-
The p21-activated kinase (PAK) family member PakD is required for chemorepulsion and proliferation inhibition by autocrine signals in Dictyostelium discoideum.PLoS One. 2014 May 5;9(5):e96633. doi: 10.1371/journal.pone.0096633. eCollection 2014. PLoS One. 2014. PMID: 24797076 Free PMC article.
-
A Dictyostelium secreted factor requires a PTEN-like phosphatase to slow proliferation and induce chemorepulsion.PLoS One. 2013;8(3):e59365. doi: 10.1371/journal.pone.0059365. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23555023 Free PMC article.
-
Cell density sensing and size determination.Dev Growth Differ. 2011 May;53(4):482-94. doi: 10.1111/j.1440-169X.2010.01248.x. Epub 2011 Apr 27. Dev Growth Differ. 2011. PMID: 21521184 Free PMC article. Review.
Cited by
-
Altered protein secretion in Batten disease.Dis Model Mech. 2021 Dec 1;14(12):dmm049152. doi: 10.1242/dmm.049152. Epub 2021 Dec 6. Dis Model Mech. 2021. PMID: 34870700 Free PMC article. Review.
-
Dipeptidyl peptidase IV is a human and murine neutrophil chemorepellent.J Immunol. 2013 Jun 15;190(12):6468-77. doi: 10.4049/jimmunol.1202583. Epub 2013 May 15. J Immunol. 2013. PMID: 23677473 Free PMC article.
-
A retinoblastoma orthologue is required for the sensing of a chalone in Dictyostelium discoideum.Eukaryot Cell. 2014 Mar;13(3):376-82. doi: 10.1128/EC.00306-13. Epub 2014 Jan 3. Eukaryot Cell. 2014. PMID: 24390142 Free PMC article.
-
An Autocrine Proliferation Repressor Regulates Dictyostelium discoideum Proliferation and Chemorepulsion Using the G Protein-Coupled Receptor GrlH.mBio. 2018 Feb 13;9(1):e02443-17. doi: 10.1128/mBio.02443-17. mBio. 2018. PMID: 29440579 Free PMC article.
-
Using Dictyostelium to Develop Therapeutics for Acute Respiratory Distress Syndrome.Front Cell Dev Biol. 2021 Jul 19;9:710005. doi: 10.3389/fcell.2021.710005. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34350188 Free PMC article. Review.
References
-
- Anand V. S., Braithwaite S. P. 2009. LRRK2 in Parkinson's disease: biochemical functions. FEBS J. 276:6428–6435 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

