The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma
- PMID: 20709804
- PMCID: PMC2928982
- DOI: 10.2353/ajpath.2010.100328
The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia, and carcinoma
Abstract
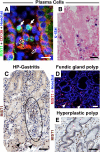
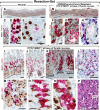
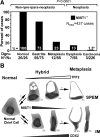
The lack of reliable molecular markers for normal differentiated epithelial cells limits understanding of human gastric carcinogenesis. Recognized precursor lesions for gastric adenocarcinoma are intestinal metaplasia and spasmolytic polypeptide expressing metaplasia (SPEM), defined here by ectopic CDX2 and TFF2 expression, respectively. In mice, expression of the bHLH transcription factor MIST1, normally restricted to mature chief cells, is down-regulated as chief cells undergo experimentally induced metaplasia. Here, we show MIST1 expression is also a specific marker of human chief cells. SPEM, with and without MIST1, is present in human lesions and, akin to murine data, likely represents transitional (TFF2(+)/MIST1(+) = "hybrid"-SPEM) and established (TFF2(+)/MIST1(-) = SPEM) stages. Co-visualization of MIST1 and CDX2 shows similar progressive loss of MIST1 with a transitional, CDX2(+)/MIST1(-) hybrid-intestinal metaplasia stage. Interinstitutional analysis and comparison of findings in tissue microarrays, resection specimens, and biopsies (n > 400 samples), comprising the entire spectrum of recognized stages of gastric carcinogenesis, confirm MIST1 expression is restricted to the chief cell compartment in normal oxyntic mucosa, rare in established metaplastic lesions, and lost in intraepithelial neoplasia/dysplasia and carcinoma of various types with the exception of rare chief cell carcinoma ( approximately 1%). Our findings implicate MIST1 as a reliable marker of mature, healthy chief cells, and we provide the first evidence that metaplasia in humans arises at least in part from the chief cell lineage.
Figures





References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Jarvi O, Lauren P. On the role of heterotopias of the intestinal epithelium in the pathogenesis of gastric cancer. Acta Pathol Microbiol Scand. 1951;29:26–44. - PubMed
-
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process; First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. - PubMed
-
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311–1315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

