Peroxisome proliferator-activated receptor {delta} regulates inflammation via NF-{kappa}B signaling in polymicrobial sepsis
- PMID: 20709805
- PMCID: PMC2947279
- DOI: 10.2353/ajpath.2010.091010
Peroxisome proliferator-activated receptor {delta} regulates inflammation via NF-{kappa}B signaling in polymicrobial sepsis
Abstract
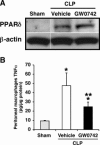
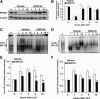
The nuclear peroxisome proliferator-activated receptor δ (PPARδ) is an important regulator of lipid metabolism. In contrast to its known effects on energy homeostasis, its biological role on inflammation is not well understood. We investigated the role of PPARδ in the modulation of the nuclear factor-κB (NF-κB)-driven inflammatory response to polymicrobial sepsis in vivo and in macrophages in vitro. We demonstrated that administration of GW0742, a specific PPARδ ligand, provided beneficial effects to rats subjected to cecal ligation and puncture, as shown by reduced systemic release of pro-inflammatory cytokines and neutrophil infiltration in lung, liver, and cecum, when compared with vehicle treatment. Molecular analysis revealed that treatment with GW0742 reduced NF-κB binding to DNA in lung and liver. In parallel experiments, heterozygous PPARδ-deficient mice suffered exaggerated lethality when subjected to cecal ligation and puncture and exhibited severe lung injury and higher levels of circulating tumor necrosis factor-α (TNFα) and keratinocyte-derived chemokine than wild-type mice. Furthermore, in lipopolysaccharide-stimulated J774.A1 macrophages, GW0742 reduced TNFα production by inhibiting NF-κB activation. RNA silencing of PPARδ abrogated the inhibitory effects of GW0742 on TNFα production. Chromatin immunoprecipitation assays revealed that PPARδ displaced the NF-κB p65 subunit from the κB elements of the TNFα promoter, while recruiting the co-repressor BCL6. These data suggest that PPARδ is a crucial anti-inflammatory regulator, providing a basis for novel sepsis therapies.
Figures


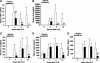







Similar articles
-
Peroxisome proliferator activator receptor-gamma ligands, 15-deoxy-Delta(12,14)-prostaglandin J2 and ciglitazone, reduce systemic inflammation in polymicrobial sepsis by modulation of signal transduction pathways.J Immunol. 2003 Dec 15;171(12):6827-37. doi: 10.4049/jimmunol.171.12.6827. J Immunol. 2003. PMID: 14662889
-
PPARdelta modulates lipopolysaccharide-induced TNFalpha inflammation signaling in cultured cardiomyocytes.J Mol Cell Cardiol. 2006 Jun;40(6):821-8. doi: 10.1016/j.yjmcc.2006.03.422. Epub 2006 May 15. J Mol Cell Cardiol. 2006. PMID: 16698033
-
Protective role of peroxisome proliferator-activated receptor-β/δ in septic shock.Am J Respir Crit Care Med. 2010 Dec 15;182(12):1506-15. doi: 10.1164/rccm.201002-0240OC. Epub 2010 Aug 6. Am J Respir Crit Care Med. 2010. PMID: 20693380
-
Thymoquinone attenuates proinflammatory responses in lipopolysaccharide-activated mast cells by modulating NF-kappaB nuclear transactivation.Biochim Biophys Acta. 2007 Apr;1770(4):556-64. doi: 10.1016/j.bbagen.2007.01.002. Epub 2007 Jan 12. Biochim Biophys Acta. 2007. PMID: 17292554
-
Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders.Cell Signal. 2013 Oct;25(10):1939-48. doi: 10.1016/j.cellsig.2013.06.007. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770291 Review.
Cited by
-
PPARs and the Development of Type 1 Diabetes.PPAR Res. 2020 Jan 9;2020:6198628. doi: 10.1155/2020/6198628. eCollection 2020. PPAR Res. 2020. PMID: 32395123 Free PMC article. Review.
-
PPAR Agonists and Metabolic Syndrome: An Established Role?Int J Mol Sci. 2018 Apr 14;19(4):1197. doi: 10.3390/ijms19041197. Int J Mol Sci. 2018. PMID: 29662003 Free PMC article. Review.
-
Selective PPARδ Agonist GW501516 Protects Against LPS-Induced Macrophage Inflammation and Acute Liver Failure in Mice via Suppressing Inflammatory Mediators.Molecules. 2024 Nov 2;29(21):5189. doi: 10.3390/molecules29215189. Molecules. 2024. PMID: 39519830 Free PMC article.
-
Investigation of PPARβ/δ within Human Dental Pulp Cells: A Preliminary In Vitro Study.PPAR Res. 2021 Mar 18;2021:8854921. doi: 10.1155/2021/8854921. eCollection 2021. PPAR Res. 2021. PMID: 33790957 Free PMC article.
-
A historical perspective on sepsis.Am J Pathol. 2012 Jul;181(1):2-7. doi: 10.1016/j.ajpath.2012.05.003. Epub 2012 May 26. Am J Pathol. 2012. PMID: 22642906 Free PMC article. Review.
References
-
- Medzhitov R, Janeway C., Jr Innate immune recognition: mechanisms and pathways. Immunol Rev. 2000;173:89–97. - PubMed
-
- Baeuerle PA. IκB-NF-κB structures: at the interface of inflammation control. Cell. 1998;11:729–731. - PubMed
-
- Zingarelli B, Sheehan M, Wong HR. Nuclear factor-κB as a therapeutic target in critical care medicine. Crit Care Med. 2003;31:S105–S111. - PubMed
-
- Brown JD, Plutzky J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation. 2007;115:518–533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

