Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking
- PMID: 20709815
- PMCID: PMC3040390
- DOI: 10.1164/rccm.201001-0126OC
Adipose stem cell treatment in mice attenuates lung and systemic injury induced by cigarette smoking
Abstract
Rationale: Adipose-derived stem cells express multiple growth factors that inhibit endothelial cell apoptosis, and demonstrate substantial pulmonary trapping after intravascular delivery.
Objectives: We hypothesized that adipose stem cells would ameliorate chronic lung injury associated with endothelial cell apoptosis, such as that occurring in emphysema.
Methods: Therapeutic effects of systemically delivered human or mouse adult adipose stem cells were evaluated in murine models of emphysema induced by chronic exposure to cigarette smoke or by inhibition of vascular endothelial growth factor receptors.
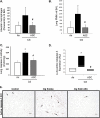
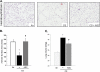
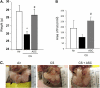
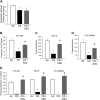
Measurements and main results: Adipose stem cells were detectable in the parenchyma and large airways of lungs up to 21 days after injection. Adipose stem cell treatment was associated with reduced inflammatory infiltration in response to cigarette smoke exposure, and markedly decreased lung cell death and airspace enlargement in both models of emphysema. Remarkably, therapeutic results of adipose stem cells extended beyond lung protection by rescuing the suppressive effects of cigarette smoke on bone marrow hematopoietic progenitor cell function, and by restoring weight loss sustained by mice during cigarette smoke exposure. Pulmonary vascular protective effects of adipose stem cells were recapitulated by application of cell-free conditioned medium, which improved lung endothelial cell repair and recovery in a wound injury repair model and antagonized effects of cigarette smoke in vitro.
Conclusions: These results suggest a useful therapeutic effect of adipose stem cells on both lung and systemic injury induced by cigarette smoke, and implicate a lung vascular protective function of adipose stem cell derived paracrine factors.
Figures


References
-
- Cai L, Johnstone BH, Cook TG, Liang Z, Traktuev D, Cornetta K, Ingram DA, Rosen ED, March KL. Suppression of hepatocyte growth factor production impairs the ability of adipose-derived stem cells to promote ischemic tissue revascularization. Stem Cells 2007;25:3234–3243. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

