APCs expressing high levels of programmed death ligand 2 sustain the development of CD4 T cell memory
- PMID: 20709947
- PMCID: PMC3057906
- DOI: 10.4049/jimmunol.1000810
APCs expressing high levels of programmed death ligand 2 sustain the development of CD4 T cell memory
Abstract
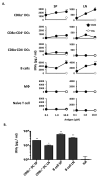
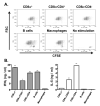
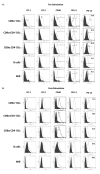
The role APCs play in the transition of T cells from effector to memory remains largely undefined. This is likely due to the low frequency at which long-lived T cells arise, which hinders analysis of the events involved in memory development. In this study, we used TCR transgenic T cells to increase the frequency of long-lived T cells and developed a transfer model suitable for defining the contribution of APCs to the development of CD4 T cell memory. Accordingly, naive TCR transgenic T cells were stimulated in vitro with Ag presented by different types of APCs and transferred into MHC class II-deficient mice for parking, and the hosts were later analyzed for long-lived T cell frequency or challenged with suboptimal dose of Ag, and the long-lived cells-driven memory responses were measured. The findings indicate that B cells and CD8alpha(+) dendritic cells sustained elevated frequencies of long-lived T cells that yielded rapid and robust memory responses upon rechallenge with suboptimal dose of Ag. Furthermore, both types of APCs had significant programmed death (PD) ligand 2 expression prior to Ag stimulation, which was maintained at a high level during presentation of Ag to T cells. Blockade of PD ligand 2 interaction with its receptor PD-1 nullified the development of memory responses. These previously unrecognized findings suggest that targeting specific APCs for Ag presentation during vaccination could prove effective against microbial infections.
Figures




References
-
- Doherty PC, Topham DJ, Tripp RA. Establishment and persistence of virus-specific CD4+ and CD8+ T cell memory. Immunol Rev. 1996;150:23–44. - PubMed
-
- Kaech SM, Wherry EJ, Ahmed R. Effector T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. - PubMed
-
- Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. - PubMed
-
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. - PubMed
-
- Van Parijs L, Abbas AK. Homeostasis and self-tolerance in the immune system: turning lymphocytes off. Science. 1998;280:243–248. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

