Novel changes in NF-{kappa}B activity during progression and regression phases of hyperplasia: role of MEK, ERK, and p38
- PMID: 20710027
- PMCID: PMC2963366
- DOI: 10.1074/jbc.M110.129353
Novel changes in NF-{kappa}B activity during progression and regression phases of hyperplasia: role of MEK, ERK, and p38
Abstract
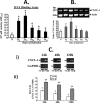
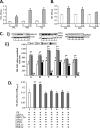
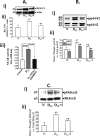
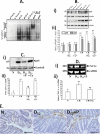
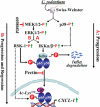
Utilizing the Citrobacter rodentium-induced transmissible murine colonic hyperplasia (TMCH) model, we measured hyperplasia and NF-κB activation during progression (days 6 and 12 post-infection) and regression (days 20-34 post-infection) phases of TMCH. NF-κB activity increased at progression in conjunction with bacterial attachment and translocation to the colonic crypts and decreased 40% by day 20. NF-κB activity at days 27 and 34, however, remained 2-3-fold higher than uninfected control. Expression of the downstream target gene CXCL-1/KC in the crypts correlated with NF-κB activation kinetics. Phosphorylation of cellular IκBα kinase (IKK)α/β (Ser(176/180)) was elevated during progression and regression of TMCH. Phosphorylation (Ser(32/36)) and degradation of IκBα, however, contributed to NF-κB activation only from days 6 to 20 but not at later time points. Phosphorylation of MEK1/2 (Ser(217/221)), ERK1/2 (Thr(202)/Tyr(204)), and p38 (Thr(180)/Tyr(182)) paralleled IKKα/β kinetics at days 6 and 12 without declining with regressing hyperplasia. siRNAs to MEK, ERK, and p38 significantly blocked NF-κB activity in vitro, whereas MEK1/2-inhibitor (PD98059) also blocked increases in MEK1/2, ERK1/2, and IKKα/β thereby inhibiting NF-κB activity in vivo. Cellular and nuclear levels of Ser(536)-phosphorylated (p65(536)) and Lys(310)-acetylated p65 subunit accompanied functional NF-κB activation during TMCH. RSK-1 phosphorylation at Thr(359)/Ser(363) in cellular/nuclear extracts and co-immunoprecipitation with cellular p65-NF-κB overlapped with p65(536) kinetics. Dietary pectin (6%) blocked NF-κB activity by blocking increases in p65 abundance and nuclear translocation thereby down-regulating CXCL-1/KC expression in the crypts. Thus, NF-κB activation persisted despite the lack of bacterial attachment to colonic mucosa beyond peak hyperplasia. The MEK/ERK/p38 pathway therefore seems to modulate sustained activation of NF-κB in colonic crypts in response to C. rodentium infection.
Figures




Similar articles
-
Citrobacter rodentium-induced NF-kappaB activation in hyperproliferating colonic epithelia: role of p65 (Ser536) phosphorylation.Br J Pharmacol. 2006 Jul;148(6):814-24. doi: 10.1038/sj.bjp.0706784. Epub 2006 Jun 5. Br J Pharmacol. 2006. PMID: 16751795 Free PMC article.
-
NF-kappaB-dependent transcriptional activation in lung carcinoma cells by farnesol involves p65/RelA(Ser276) phosphorylation via the MEK-MSK1 signaling pathway.J Biol Chem. 2008 Jun 13;283(24):16391-9. doi: 10.1074/jbc.M800945200. Epub 2008 Apr 18. J Biol Chem. 2008. PMID: 18424438 Free PMC article.
-
Distinct compartmentalization of NF-κB activity in crypt and crypt-denuded lamina propria precedes and accompanies hyperplasia and/or colitis following bacterial infection.Infect Immun. 2012 Feb;80(2):753-67. doi: 10.1128/IAI.06101-11. Epub 2011 Dec 5. Infect Immun. 2012. PMID: 22144489 Free PMC article.
-
Intrinsic and acquired resistance to MEK1/2 inhibitors in cancer.Biochem Soc Trans. 2014 Aug;42(4):776-83. doi: 10.1042/BST20140129. Biochem Soc Trans. 2014. PMID: 25109957 Review.
-
Reining in nuclear factor-kappaB in skeletal muscle disorders.Curr Opin Clin Nutr Metab Care. 2013 May;16(3):251-7. doi: 10.1097/MCO.0b013e3283600e79. Curr Opin Clin Nutr Metab Care. 2013. PMID: 23493016 Review.
Cited by
-
Infection-induced signals generated at the plasma membrane epigenetically regulate Wnt signaling in vitro and in vivo.J Biol Chem. 2020 Jan 24;295(4):1021-1035. doi: 10.1074/jbc.RA119.010285. Epub 2019 Dec 13. J Biol Chem. 2020. PMID: 31836665 Free PMC article.
-
Dietary Interventions Ameliorate Infectious Colitis by Restoring the Microbiome and Promoting Stem Cell Proliferation in Mice.Int J Mol Sci. 2021 Dec 29;23(1):339. doi: 10.3390/ijms23010339. Int J Mol Sci. 2021. PMID: 35008767 Free PMC article.
-
Requirement of epithelial integrin-linked kinase for facilitation of Citrobacter rodentium-induced colitis.BMC Gastroenterol. 2013 Sep 11;13:137. doi: 10.1186/1471-230X-13-137. BMC Gastroenterol. 2013. PMID: 24024606 Free PMC article.
-
DCLK1 isoforms and aberrant Notch signaling in the regulation of human and murine colitis.Cell Death Discov. 2021 Jun 17;7(1):169. doi: 10.1038/s41420-021-00526-9. Cell Death Discov. 2021. PMID: 34226497 Free PMC article.
-
DCLK1 Regulates Tumor Stemness and Cisplatin Resistance in Non-small Cell Lung Cancer via ABCD-Member-4.Mol Ther Oncolytics. 2020 May 27;18:24-36. doi: 10.1016/j.omto.2020.05.012. eCollection 2020 Sep 25. Mol Ther Oncolytics. 2020. PMID: 32637578 Free PMC article.
References
-
- Risio M., Lipkin M., Candelaresi G., Bertone A., Coverlizza S., Rossini F. P. (1991) Cancer Res. 51, 1917–1921 - PubMed
-
- Vogelstein B., Kinzler K. W. (1994) Cold Spring Harbor Symp. Quant. Biol. 59, 517–521 - PubMed
-
- Peifer M. (1995) Trends Cell Biol. 5, 224–229 - PubMed
-
- Stewart D. B., Nelson W. J. (1997) J. Biol. Chem. 272, 29652–29662 - PubMed
-
- Blobe G. C., Obeid L. M., Hannun Y. A. (1994) Cancer Metastasis Rev. 13, 411–431 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

