Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells
- PMID: 20710042
- PMCID: PMC2981028
- DOI: 10.1158/0008-5472.CAN-10-0052
Type 1 insulin-like growth factor receptor translocates to the nucleus of human tumor cells
Abstract
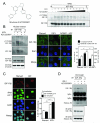
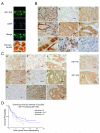
The type 1 insulin-like growth factor receptor (IGF-1R) is a transmembrane glycoprotein composed of two extracellular alpha subunits and two beta subunits with tyrosine kinase activity. The IGF-1R is frequently upregulated in cancers and signals from the cell surface to promote proliferation and cell survival. Recent attention has focused on the IGF-1R as a target for cancer treatment. Here, we report that the nuclei of human tumor cells contain IGF-1R, detectable using multiple antibodies to alpha- and beta-subunit domains. Cell-surface IGF-1R translocates to the nucleus following clathrin-mediated endocytosis, regulated by IGF levels. The IGF-1R is unusual among transmembrane receptors that undergo nuclear import, in that both alpha and beta subunits traffic to the nucleus. Nuclear IGF-1R is phosphorylated in response to ligand and undergoes IGF-induced interaction with chromatin, suggesting direct engagement in transcriptional regulation. The IGF dependence of these phenomena indicates a requirement for the receptor kinase, and indeed, IGF-1R nuclear import and chromatin binding can be blocked by a novel IGF-1R kinase inhibitor. Nuclear IGF-1R is detectable in primary renal cancer cells, formalin-fixed tumors, preinvasive lesions in the breast, and nonmalignant tissues characterized by a high proliferation rate. In clear cell renal cancer, nuclear IGF-1R is associated with adverse prognosis. Our findings suggest that IGF-1R nuclear import has biological significance, may contribute directly to IGF-1R function, and may influence the efficacy of IGF-1R inhibitory drugs.
(c)2010 AACR.
Figures


References
-
- Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14:6364–70. - PubMed
-
- Romanelli RJ, LeBeau AP, Fulmer CG, Lazzarino DA, Hochberg A, Wood TL. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. J Biol Chem. 2007;282:22513–24. - PubMed
-
- Lin SY, Makino K, Xia W, et al. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat Cell Biol. 2001;3:802–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

