Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities
- PMID: 20711184
- PMCID: PMC2928860
- DOI: 10.1038/nn.2617
Rod photoreceptors drive circadian photoentrainment across a wide range of light intensities
Abstract
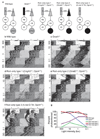
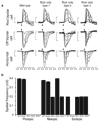
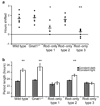
In mammals, synchronization of the circadian pacemaker in the hypothalamus is achieved through direct input from the eyes conveyed by intrinsically photosensitive retinal ganglion cells (ipRGCs). Circadian photoentrainment can be maintained by rod and cone photoreceptors, but their functional contributions and their retinal circuits that impinge on ipRGCs are not well understood. Using mice that lack functional rods or in which rods are the only functional photoreceptors, we found that rods were solely responsible for photoentrainment at scotopic light intensities. Rods were also capable of driving circadian photoentrainment at photopic intensities at which they were incapable of supporting a visually guided behavior. Using mice in which cone photoreceptors were ablated, we found that rods signal through cones at high light intensities, but not at low light intensities. Thus, rods use two distinct retinal circuits to drive ipRGC function to support circadian photoentrainment across a wide range of light intensities.
Figures
References
-
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

