Oncogenic function of the MUC1 receptor subunit in gene regulation
- PMID: 20711235
- PMCID: PMC3615539
- DOI: 10.1038/onc.2010.334
Oncogenic function of the MUC1 receptor subunit in gene regulation
Abstract
The mucin 1 (MUC1) oncoprotein is overexpressed by diverse human cancers; however, it has remained largely unclear how MUC1 contributes to tumorigenesis. In this issue of Oncogene and in concert with published work, Behrens et al. report that the MUC1 receptor subunit activates genes involved in invasion, angiogenesis and metastasis.
Figures
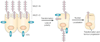

Comment on
-
The reactive tumor microenvironment: MUC1 signaling directly reprograms transcription of CTGF.Oncogene. 2010 Oct 21;29(42):5667-77. doi: 10.1038/onc.2010.327. Epub 2010 Aug 9. Oncogene. 2010. PMID: 20697347 Free PMC article.
References
-
- Deng YZ, Chen PP, Wang Y, Yin D, Koeffler HP, Li B, et al. Connective tissue growth factor is overexpressed in esophageal squamous cell carcinoma and promotes tumorigenicity through beta-catenin-T-cell factor/Lef signaling. J. Biol. Chem. 2007;282:36571–36581. - PubMed
-
- Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3beta-mediated phosphorylation and degradation of beta-catenin. Cancer Res. 2005;65:10413–10422. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

