Efficacy and safety of citalopram versus amitriptyline in the treatment of major depression
- PMID: 20711288
- PMCID: PMC2918306
- DOI: 10.4103/0019-5545.55952
Efficacy and safety of citalopram versus amitriptyline in the treatment of major depression
Abstract
Background: Double-blind clinical trials comparing citalopram with amitriptyline or other tricyclic antidepressants are lacking in India.
Aim: To evaluate the efficacy and safety of the newer antidepressant citalopram in the treatment of major depression.
Methods: The clinical acceptability and safety profile of citalopram was assessed and compared with that of amitriptyline in 40 patients in an outpatient set-up. Patients aged 18 to 65 years who fulfilled the diagnostic criteria for a single or recurrent major depressive disorder (as defined by DSM-IV) for a minimum of 2 weeks were enrolled. Patient assessment was done at screening, baseline, end of week 1, week 2, week 3, week 4, week 5 and week 6 for efficacy and safety parameters such as Hamilton Depression Rating Scale (HDRS), Clinical Global Impression (CGI) Scale, adverse event follow up, blood pressure and pulse. Three-level statistical analysis including ANOVA was performed on all efficacy measures.
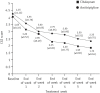
Results: On the HDRS the percentage reduction in the mean score for the citalopram group (Group 1) was 72.12%, while that for the amitriptyline group (Group 2) was 67.93%. On the CGI-Improvement Scale, the percentage reduction at the end of the study for the citalopram group was 56.79% while in the amitriptyline group it was 44.70%. Twenty per cent of patients in Group 1 reported adverse events compared to 75% in Group 2.
Conclusions: Citalopram is effective in the treatment of major depression at the dosages range of 20-60 mg/day and its efficacy is equivalent to that of standard tricyclic antidepressants such as amitriptyline, with a substantially better tolerability profile.
Keywords: Major depression; SSRIs; side-effects; tricyclic antidepressants.
Figures
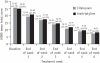
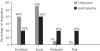
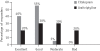
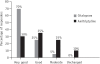
Similar articles
-
A comparative study of the efficacy and safety of mirtazapine versus amitriptyline in the treatment of major depression.Indian J Psychiatry. 2002 Jul;44(3):260-5. Indian J Psychiatry. 2002. PMID: 21206581 Free PMC article.
-
Comparison of the tolerability and efficacy of citalopram and amitriptyline in elderly depressed patients treated in general practice.Depress Anxiety. 1998;8(4):147-53. Depress Anxiety. 1998. PMID: 9871816 Clinical Trial.
-
Efficacy and tolerability of mirtazapine versus citalopram: a double-blind, randomized study in patients with major depressive disorder. Nordic Antidepressant Study Group.Int Clin Psychopharmacol. 1999 Nov;14(6):329-37. doi: 10.1097/00004850-199911000-00002. Int Clin Psychopharmacol. 1999. PMID: 10565799 Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Comparative efficacy of antidepressants.Drugs. 1992;43 Suppl 2:11-22; discussion 22-3. doi: 10.2165/00003495-199200432-00004. Drugs. 1992. PMID: 1378369 Review.
Cited by
-
Amitriptyline and Sexual Function: A Systematic Review Updated for Sexual Health Practice.Am J Mens Health. 2018 Mar;12(2):370-379. doi: 10.1177/1557988317734519. Epub 2017 Oct 11. Am J Mens Health. 2018. PMID: 29019272 Free PMC article.
-
Research on antidepressants in India.Indian J Psychiatry. 2010 Jan;52(Suppl 1):S341-54. doi: 10.4103/0019-5545.69263. Indian J Psychiatry. 2010. PMID: 21836704 Free PMC article.
-
"Modified Schirmer Test in Assessment of Salivary Flow Rate Among Patients on Antidepressants": A Comparative Study.J Int Soc Prev Community Dent. 2021 Jun 10;11(3):287-293. doi: 10.4103/jispcd.JISPCD_416_20. eCollection 2021 May-Jun. J Int Soc Prev Community Dent. 2021. PMID: 34268191 Free PMC article.
-
A systematic review and meta-analysis of trials of treatment of depression from India.Indian J Psychiatry. 2014 Jan;56(1):29-38. doi: 10.4103/0019-5545.124711. Indian J Psychiatry. 2014. PMID: 24574556 Free PMC article.
-
Indian Journal of Psychiatry and psychiatric research in India: Past, Present and Future.Indian J Psychiatry. 2010 Jan;52(Suppl 1):S13-8. doi: 10.4103/0019-5545.69197. Indian J Psychiatry. 2010. PMID: 21836669 Free PMC article.
References
-
- Hyttel J. Citalopram—pharmacological profile of a specific serotonin uptake inhibitor with antidepressant activity. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:277–95. - PubMed
-
- Lindegaard PO, Kragh SP, Bjette M, et al. Citalopram, a selected serotonin reuptake inhibitor: Clinical antidepressive and long term effect—a phase II study. Psychopharmacology. 1984;77:199–204. - PubMed
-
- Gastpar M, Gastpar G. Preliminary studies with citalopram (LU 10-171), a specific 5-HT-reuptake inhibitor, as antidepressant. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:319–25. - PubMed
-
- Ofsti E. Citalopram—a specific 5-HT-reuptake inhibitor—as an antidepressant drug: A phase II multicentre trail. Prog Neuropsychopharmacol Biol Psychiatry. 1982;6:327–35. - PubMed

