Positive feedback regulation between phospholipase D and Wnt signaling promotes Wnt-driven anchorage-independent growth of colorectal cancer cells
- PMID: 20711340
- PMCID: PMC2920823
- DOI: 10.1371/journal.pone.0012109
Positive feedback regulation between phospholipase D and Wnt signaling promotes Wnt-driven anchorage-independent growth of colorectal cancer cells
Abstract
Background: Aberrant activation of the canonical Wnt/beta-catenin pathway occurs in almost all colorectal cancers and contributes to their growth, invasion and survival. Phopholipase D (PLD) has been implicated in progression of colorectal carcinoma However, an understanding of the targets and regulation of this important pathway remains incomplete and besides, relationship between Wnt signaling and PLD is not known.
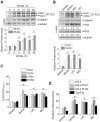
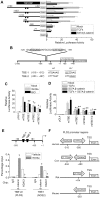
Methodology/principal findings: Here, we demonstrate that PLD isozymes, PLD1 and PLD2 are direct targets and positive feedback regulators of the Wnt/beta-catenin signaling. Wnt3a and Wnt mimetics significantly enhanced the expression of PLDs at a transcriptional level in HCT116 colorectal cancer cells, whereas silencing of beta-catenin gene expression or utilization of a dominant negative form of T cell factor-4 (TCF-4) inhibited expression of PLDs. Moreover, both PLD1 and PLD2 were highly induced in colon, liver and stomach tissues of mice after injection of LiCl, a Wnt mimetic. Wnt3a stimulated formation of the beta-catenin/TCF complexes to two functional TCF-4-binding elements within the PLD2 promoter as assessed by chromatin immunoprecipitation assay. Suppressing PLD using gene silencing or selective inhibitor blocked the ability of beta-catenin to transcriptionally activate PLD and other Wnt target genes by preventing formation of the beta-catenin/TCF-4 complex, whereas tactics to elevate intracellular levels of phosphatidic acid, the product of PLD activity, enhanced these effects. Here we show that PLD is necessary for Wnt3a-driven invasion and anchorage-independent growth of colon cancer cells.
Conclusion/significance: PLD isozyme acts as a novel transcriptional target and positive feedback regulator of Wnt signaling, and then promotes Wnt-driven anchorage-independent growth of colorectal cancer cells. We propose that therapeutic interventions targeting PLD may confer a clinical benefit in Wnt/beta-catenin-driven malignancies.
Conflict of interest statement
Figures




Similar articles
-
Phospholipase D1 drives a positive feedback loop to reinforce the Wnt/beta-catenin/TCF signaling axis.Cancer Res. 2010 May 15;70(10):4233-42. doi: 10.1158/0008-5472.CAN-09-3470. Epub 2010 May 4. Cancer Res. 2010. PMID: 20442281
-
HEF1, a novel target of Wnt signaling, promotes colonic cell migration and cancer progression.Oncogene. 2011 Jun 9;30(23):2633-43. doi: 10.1038/onc.2010.632. Epub 2011 Feb 14. Oncogene. 2011. PMID: 21317929 Free PMC article.
-
Phospholipase D1 Inhibition Linked to Upregulation of ICAT Blocks Colorectal Cancer Growth Hyperactivated by Wnt/β-Catenin and PI3K/Akt Signaling.Clin Cancer Res. 2017 Dec 1;23(23):7340-7350. doi: 10.1158/1078-0432.CCR-17-0749. Epub 2017 Sep 22. Clin Cancer Res. 2017. PMID: 28939743
-
Phospholipase D meets Wnt signaling: a new target for cancer therapy.Cancer Res. 2011 Jan 15;71(2):293-7. doi: 10.1158/0008-5472.CAN-10-2463. Epub 2011 Jan 11. Cancer Res. 2011. PMID: 21224347 Review.
-
Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer.Cells. 2020 Sep 19;9(9):2125. doi: 10.3390/cells9092125. Cells. 2020. PMID: 32961708 Free PMC article. Review.
Cited by
-
Hypoxia-inducible factor 1-alpha up-regulates the expression of phospholipase D2 in colon cancer cells under hypoxic conditions.Med Oncol. 2015 Jan;32(1):394. doi: 10.1007/s12032-014-0394-9. Epub 2014 Nov 29. Med Oncol. 2015. PMID: 25432699
-
Positive feedback loop of hepatoma-derived growth factor and β-catenin promotes carcinogenesis of colorectal cancer.Oncotarget. 2015 Oct 6;6(30):29357-74. doi: 10.18632/oncotarget.4982. Oncotarget. 2015. PMID: 26296979 Free PMC article.
-
Wnt proteins.Cold Spring Harb Perspect Biol. 2012 Sep 1;4(9):a007864. doi: 10.1101/cshperspect.a007864. Cold Spring Harb Perspect Biol. 2012. PMID: 22952392 Free PMC article. Review.
-
A dynamic exchange of TCF3 and TCF4 transcription factors controls MYC expression in colorectal cancer cells.Cell Cycle. 2015;14(3):323-32. doi: 10.4161/15384101.2014.980643. Cell Cycle. 2015. PMID: 25659031 Free PMC article.
-
High Expression of PhospholipaseD2 Induced by Hypoxia Promotes Proliferation of Colon Cancer Cells through Activating NF- κ Bp65 Signaling Pathway.Pathol Oncol Res. 2020 Jan;26(1):281-290. doi: 10.1007/s12253-018-0429-1. Epub 2018 Aug 8. Pathol Oncol Res. 2020. PMID: 30091007
References
-
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. - PubMed
-
- Morin PJ. β-Catenin signaling and cancer. Bioassays. 1999;21:1021–1030. - PubMed
-
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

