Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus
- PMID: 20711354
- PMCID: PMC2920837
- DOI: 10.1371/journal.pone.0012137
Host immune response to mosquito-transmitted chikungunya virus differs from that elicited by needle inoculated virus
Abstract
Background: Mosquito-borne diseases are a worldwide public health threat. Mosquitoes transmit viruses or parasites during feeding, along with salivary proteins that modulate host responses to facilitate both blood feeding and pathogen transmission. Understanding these earliest events in mosquito transmission of arboviruses by mosquitoes is essential for development and assessment of rational vaccine and treatment strategies. In this report, we compared host immune responses to chikungunya virus (CHIKV) transmission by (1) mosquito bite, or (2) by needle inoculation.
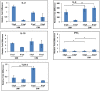
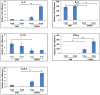
Methods and findings: Differential cytokine expression was measured using quantitative real-time RT-PCR, at sites of uninfected mosquito bites, CHIKV-infected mosquito bites, and needle-inoculated CHIKV. Both uninfected and CHIKV infected mosquitoes polarized host cytokine response to a TH2 profile. Compared to uninfected mosquito bites, expression of IL-4 induced by CHIKV-infected mosquitoes were 150 fold and 527.1 fold higher at 3 hours post feeding (hpf) and 6 hpf, respectively. A significant suppression of TH1 cytokines and TLR-3 was also observed. These significant differences may result from variation in the composition of uninfected and CHIKV-infected mosquito saliva. Needle injected CHIKV induced a robust interferon-gamma, no detectable IL-4, and a significant up-regulation of TLR-3.
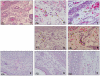
Conclusions: This report describes the first analysis of cutaneous cytokines in mice bitten by CHIKV-infected mosquitoes. Our data demonstrate contrasting immune activation in the response to CHIKV infection by mosquito bite or needle inoculation. The significant role of mosquito saliva in these earliest events of CHIKV transmission and infection are highlighted.
Conflict of interest statement
Figures
Similar articles
-
Mosquito saliva induced cutaneous events augment Chikungunya virus replication and disease progression.Infect Genet Evol. 2016 Jun;40:126-135. doi: 10.1016/j.meegid.2016.02.033. Epub 2016 Feb 27. Infect Genet Evol. 2016. PMID: 26925703
-
Assessment of the transmission of live-attenuated chikungunya virus vaccine VLA1553 by Aedes albopictus mosquitoes.Parasit Vectors. 2025 May 12;18(1):171. doi: 10.1186/s13071-025-06789-w. Parasit Vectors. 2025. PMID: 40355954 Free PMC article.
-
Mosquito co-infection with Zika and chikungunya virus allows simultaneous transmission without affecting vector competence of Aedes aegypti.PLoS Negl Trop Dis. 2017 Jun 1;11(6):e0005654. doi: 10.1371/journal.pntd.0005654. eCollection 2017 Jun. PLoS Negl Trop Dis. 2017. PMID: 28570693 Free PMC article.
-
Immune Response to Chikungunya Virus: Sex as a Biological Variable and Implications for Natural Delivery via the Mosquito.Viruses. 2023 Sep 3;15(9):1869. doi: 10.3390/v15091869. Viruses. 2023. PMID: 37766276 Free PMC article. Review.
-
The new European invader Aedes (Finlaya) koreicus: a potential vector of chikungunya virus.Pathog Glob Health. 2018 May;112(3):107-114. doi: 10.1080/20477724.2018.1464780. Epub 2018 May 8. Pathog Glob Health. 2018. PMID: 29737236 Free PMC article. Review.
Cited by
-
The tortoise or the hare? Impacts of within-host dynamics on transmission success of arthropod-borne viruses.Philos Trans R Soc Lond B Biol Sci. 2015 Aug 19;370(1675):20140299. doi: 10.1098/rstb.2014.0299. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 26150665 Free PMC article. Review.
-
Activation of the innate immune response against DENV in normal non-transformed human fibroblasts.PLoS Negl Trop Dis. 2011 Dec;5(12):e1420. doi: 10.1371/journal.pntd.0001420. Epub 2011 Dec 20. PLoS Negl Trop Dis. 2011. PMID: 22206025 Free PMC article.
-
Aedes aegypti salivary protein "aegyptin" co-inoculation modulates dengue virus infection in the vertebrate host.Virology. 2014 Nov;468-470:133-139. doi: 10.1016/j.virol.2014.07.019. Epub 2014 Aug 28. Virology. 2014. PMID: 25173089 Free PMC article.
-
Complex Roles of Neutrophils during Arboviral Infections.Cells. 2021 May 26;10(6):1324. doi: 10.3390/cells10061324. Cells. 2021. PMID: 34073501 Free PMC article. Review.
-
Route of inoculation and mosquito vector exposure modulate dengue virus replication kinetics and immune responses in rhesus macaques.PLoS Negl Trop Dis. 2020 Apr 8;14(4):e0008191. doi: 10.1371/journal.pntd.0008191. eCollection 2020 Apr. PLoS Negl Trop Dis. 2020. PMID: 32267846 Free PMC article.
References
-
- Gratz NG. Emerging and resurging vector-borne diseases. Annu Rev Entomol. 1999;44:51–75. - PubMed
-
- Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33:330–342. - PubMed
-
- Simon F, Savini H, Parola P. Chikungunya: a paradigm of emergence and globalization of vector-borne diseases. Med Clin North Am. 2008;92:1323–1343. - PubMed
-
- Hochedez P, Hausfater P, Jaureguiberry S, Gay F, Datry A, et al. Cases of chikungunya fever imported from the islands of the South West Indian Ocean to Paris, France. Euro Surveill. 2007;12
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

