The Mycobacterium tuberculosis proteasome active site threonine is essential for persistence yet dispensable for replication and resistance to nitric oxide
- PMID: 20711362
- PMCID: PMC2920845
- DOI: 10.1371/journal.ppat.1001040
The Mycobacterium tuberculosis proteasome active site threonine is essential for persistence yet dispensable for replication and resistance to nitric oxide
Abstract
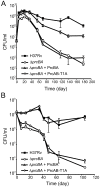
Previous work revealed that conditional depletion of the core proteasome subunits PrcB and PrcA impaired growth of Mycobacterium tuberculosis in vitro and in mouse lungs, caused hypersusceptibility to nitric oxide (NO) and impaired persistence of the bacilli during chronic mouse infections. Here, we show that genetic deletion of prcBA led to similar phenotypes. Surprisingly, however, an active site mutant proteasome complemented the in vitro and in vivo growth defects of the prcBA knockout (Delta prcBA) as well as its NO hypersensitivity. In contrast, long-term survival of M. tuberculosis in stationary phase and during starvation in vitro and in the chronic phase of mouse infection required a proteolytically active proteasome. Inhibition of inducible nitric oxide synthase did not rescue survival of Delta prcBA, revealing a function beyond NO defense, by which the proteasome contributes to M. tuberculosis fitness during chronic mouse infections. These findings suggest that proteasomal proteolysis facilitates mycobacterial persistence, that M. tuberculosis faces starvation during chronic mouse infections and that the proteasome serves a proteolysis-independent function.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Arrigo AP, Tanaka K, Goldberg AL, Welch WJ. Identity of the 19S ‘prosome’ particle with the large multifunctional protease complex of mammalian cells (the proteasome). Nature. 1988;331:192–194. - PubMed
-
- Baumeister W, Dahlmann B, Hegerl R, Kopp F, Kuehn L, et al. Electron microscopy and image analysis of the multicatalytic proteinase. FEBS Lett. 1988;241:239–245. - PubMed
-
- Kisselev AF, Songyang Z, Goldberg AL. Why does threonine, and not serine, function as the active site nucleophile in proteasomes? J Biol Chem. 2000;275:14831–14837. - PubMed
-
- Lupas A, Zuhl F, Tamura T, Wolf S, Nagy I, et al. Eubacterial proteasomes. Mol Biol Rep. 1997;24:125–131. - PubMed
-
- De Mot R, Nagy I, Walz J, Baumeister W. Proteasomes and other self-compartmentalizing proteases in prokaryotes. Trends Microbiol. 1999;7:88–92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

