Widespread protein aggregation as an inherent part of aging in C. elegans
- PMID: 20711477
- PMCID: PMC2919420
- DOI: 10.1371/journal.pbio.1000450
Widespread protein aggregation as an inherent part of aging in C. elegans
Abstract
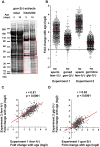
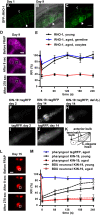
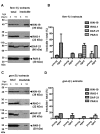
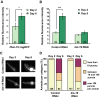
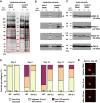
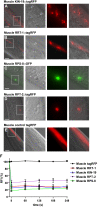
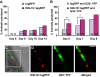
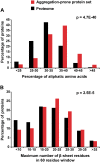
Aberrant protein aggregation is a hallmark of many age-related diseases, yet little is known about whether proteins aggregate with age in a non-disease setting. Using a systematic proteomics approach, we identified several hundred proteins that become more insoluble with age in the multicellular organism Caenorhabditis elegans. These proteins are predicted to be significantly enriched in beta-sheets, which promote disease protein aggregation. Strikingly, these insoluble proteins are highly over-represented in aggregates found in human neurodegeneration. We examined several of these proteins in vivo and confirmed their propensity to aggregate with age. Different proteins aggregated in different tissues and cellular compartments. Protein insolubility and aggregation were significantly delayed or even halted by reduced insulin/IGF-1-signaling, which also slows aging. We found a significant overlap between proteins that become insoluble and proteins that influence lifespan and/or polyglutamine-repeat aggregation. Moreover, overexpressing one aggregating protein enhanced polyglutamine-repeat pathology. Together our findings indicate that widespread protein insolubility and aggregation is an inherent part of aging and that it may influence both lifespan and neurodegenerative disease.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Comment in
-
Protein aggregation increases with age.PLoS Biol. 2010 Aug 10;8(8):e1000449. doi: 10.1371/journal.pbio.1000449. PLoS Biol. 2010. PMID: 20711479 Free PMC article. No abstract available.
References
-
- Cuervo A. M, Dice J. F. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275:31505–31513. - PubMed
-
- Lund J, Tedesco P, Duke K, Wang J, Kim S. K, et al. Transcriptional profile of aging in C. elegans. Curr Biol. 2002;12:1566–1573. - PubMed
-
- Poon H. F, Vaishnav R. A, Getchell T. V, Getchell M. L, Butterfield D. A. Quantitative proteomics analysis of differential protein expression and oxidative modification of specific proteins in the brains of old mice. Neurobiol Aging. 2006;27:1010–1019. - PubMed
-
- Squier T. C. Oxidative stress and protein aggregation during biological aging. Exp Gerontol. 2001;36:1539–1550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

