The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial
- PMID: 20711481
- PMCID: PMC2919424
- DOI: 10.1371/journal.pmed.1000321
The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial
Abstract
Background: Most HIV-1-infected patients on effective antiretroviral therapy (ART) with plasma HIV-1 RNA levels below the detection limits of commercial assays have residual viremia measurable by more sensitive methods. We assessed whether adding raltegravir lowered the level of residual viremia in such patients.
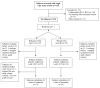
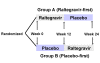
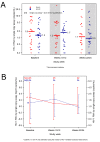
Methods and findings: Patients receiving ART who had plasma HIV-1 RNA levels below 50 copies/mL but detectable viremia by single copy assay (SCA) were randomized to add either raltegravir or placebo to their ART regimen for 12 weeks; patients then crossed-over to the other therapy for an additional 12 weeks while continuing pre-study ART. The primary endpoint was the plasma HIV-1 RNA by SCA averaged between weeks 10 and 12 (10/12) compared between treatment groups. Fifty-three patients were enrolled. The median screening HIV-1 RNA was 1.7 copies/mL. The HIV-1 RNA level at weeks 10/12 did not differ significantly between the raltegravir-intensified (n = 25) and the placebo (n = 24) groups (median 1.2 versus 1.7 copies/mL, p = 0.55, Wilcoxon rank sum test), nor did the change in HIV-1 RNA level from baseline to week 10/12 (median -0.2 and -0.1 copies/mL, p = 0.71, Wilcoxon rank sum test). There was also no significant change in HIV-1 RNA level from weeks 10/12 to weeks 22/24 after patients crossed-over. There was a greater CD4 cell count increase from baseline to week 12 in the raltegravir-intensified group compared with the placebo group (+42 versus -44 cells/mm(3), p = 0.082, Wilcoxon rank sum test), which reversed after the cross-over. This CD4 cell count change was not associated with an effect of raltegravir intensification on markers of CD4 or CD8 cell activation in blood.
Conclusion: In this randomized, double-blind cross-over study, 12 weeks of raltegravir intensification did not demonstrably reduce low-level plasma viremia in patients on currently recommended ART. This finding suggests that residual viremia does not arise from ongoing cycles of HIV-1 replication and infection of new cells. New therapeutic strategies to eliminate reservoirs that produce residual viremia will be required to eradicate HIV-1 infection.
Trial registration: ClinicalTrials.gov NCT00515827
Conflict of interest statement
JWM: Gilead Sciences (consultant); Merck (consultant and grant support); Chimerix (consultant); RFS Pharmaceuticals (owns stock options). JJE: Merck, GlaxoSmithKline (consultant and grant support), Bristol-Myers Squibb, Tibotec, Chimerix, Avexa, and Tobira (consultant). RTG: Tibotec (research grant support) and Gilead (grant support). DM: Merck, Bristol-Myers Squibb, and GlaxoSmithKline (consultant and research support); Gilead Sciences (research support and common stock); Chimerix (consultant); Roche, Trimeris, and Tibotec (research support).
Figures
References
-
- Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–1632. - PubMed
-
- Coiras M, Lopez-Huertas MR, Perez-Olmeda M, Alcami J. Understanding HIV-1 latency provides clues for the eradication of long-term reservoirs. Nat Rev Microbiol. 2009;7:798–812. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069424/AI/NIAID NIH HHS/United States
- UL1 RR025780/RR/NCRR NIH HHS/United States
- U01 AI069532/AI/NIAID NIH HHS/United States
- 25XS119/PHS HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI069495/AI/NIAID NIH HHS/United States
- U01 AI069511/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States
- U01 AI069502/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- U01 AI069423/AI/NIAID NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- UM1 AI069434/AI/NIAID NIH HHS/United States
- UL1 RR024996/RR/NCRR NIH HHS/United States
- AI 068634/AI/NIAID NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI069495/AI/NIAID NIH HHS/United States
- U01 AI069484/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069556/AI/NIAID NIH HHS/United States
- M01 RR000044/RR/NCRR NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AI069501/AI/NIAID NIH HHS/United States
- 5U01A1068636-04/PHS HHS/United States
- U01 AI069450/AI/NIAID NIH HHS/United States
- UM1 AI069556/AI/NIAID NIH HHS/United States
- M01 RR000032/RR/NCRR NIH HHS/United States
- UM1 AI069450/AI/NIAID NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- UM1 AI069532/AI/NIAID NIH HHS/United States
- U01 AI069424/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- U01 AI069494/AI/NIAID NIH HHS/United States
- U01AI068636/AI/NIAID NIH HHS/United States
- U01 AI069452/AI/NIAID NIH HHS/United States
- U01 AI069471/AI/NIAID NIH HHS/United States
- U01 AI069472/AI/NIAID NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

