AAV's anatomy: roadmap for optimizing vectors for translational success
- PMID: 20712583
- PMCID: PMC3920455
- DOI: 10.2174/156652310793180706
AAV's anatomy: roadmap for optimizing vectors for translational success
Abstract
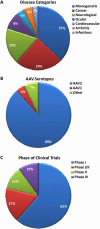
Adeno-Associated Virus based vectors (rAAV) are advantageous for human gene therapy due to low inflammatory responses, lack of toxicity, natural persistence, and ability to transencapsidate the genome allowing large variations in vector biology and tropism. Over sixty clinical trials have been conducted using rAAV serotype 2 for gene delivery with a number demonstrating success in immunoprivileged sites, including the retina and the CNS. Furthermore, an increasing number of trials have been initiated utilizing other serotypes of AAV to exploit vector tropism, trafficking, and expression efficiency. While these trials have demonstrated success in safety with emerging success in clinical outcomes, one benefit has been identification of issues associated with vector administration in humans (e.g. the role of pre-existing antibody responses, loss of transgene expression in non-immunoprivileged sites, and low transgene expression levels). For these reasons, several strategies are being used to optimize rAAV vectors, ranging from addition of exogenous agents for immune evasion to optimization of the transgene cassette for enhanced therapeutic output. By far, the vast majority of approaches have focused on genetic manipulation of the viral capsid. These methods include rational mutagenesis, engineering of targeting peptides, generation of chimeric particles, library and directed evolution approaches, as well as immune evasion modifications. Overall, these modifications have created a new repertoire of AAV vectors with improved targeting, transgene expression, and immune evasion. Continued work in these areas should synergize strategies to improve capsids and transgene cassettes that will eventually lead to optimized vectors ideally suited for translational success.
Figures
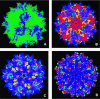
 , AAVx;
, AAVx;  , AAVy. B. Example of a chimeric virus (adapted from chimeric-1829 [195]).
, AAVy. B. Example of a chimeric virus (adapted from chimeric-1829 [195]).  , AAV1;
, AAV1;  , AAV8;
, AAV8;  , AAV2;
, AAV2;  , AAV9. C. Combinatorial representation of human and murine antibody and cell mediated epitopes of AAV serotypes displayed on an AAV2 background.
, AAV9. C. Combinatorial representation of human and murine antibody and cell mediated epitopes of AAV serotypes displayed on an AAV2 background.  ,
,  ,
,  , [243];
, [243];  , [246]; ○, [244];
, [246]; ○, [244];  , [238];
, [238];  , [245];
, [245];  , [247];
, [247];  , AAV2 background. D. Capsid modifications rendering ability to escape neutralization displayed on an AAV2 background.
, AAV2 background. D. Capsid modifications rendering ability to escape neutralization displayed on an AAV2 background.  , [264];
, [264];  , [162];
, [162];  , [208];
, [208];  , [263];
, [263];  , AAV2 background. The available structure of the AAV2 monomer (PDB accession no. 1LP3) was supplied as a template for capsid generation. Capsid features were rendered using PyMOL (
, AAV2 background. The available structure of the AAV2 monomer (PDB accession no. 1LP3) was supplied as a template for capsid generation. Capsid features were rendered using PyMOL (References
-
- Kawabata K, Takakura Y, Hashida M. The fate of plasmid DNA after intravenous injection in mice: involvement of scavenger receptors in its hepatic uptake. Pharm Res. 1995;12:825–30. - PubMed
-
- Soutschek J, Akinc A, Bramlage B, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–8. - PubMed
-
- Wooddell CI, Reppen T, Wolff JA, Herweijer H. Sustained liver-specific transgene expression from the albumin promoter in mice following hydrodynamic plasmid DNA delivery. J Gene Med. 2008;10:551–63. - PubMed
-
- Itaka K, Kataoka K. Recent development of nonviral gene delivery systems with virus-like structures and mechanisms. Eur J Pharm Biopharm. 2009;71:475–83. - PubMed
-
- Barteau B, Chevre R, Letrou-Bonneval E, Labas R, Lambert O, Pitard B. Physicochemical parameters of non-viral vectors that govern transfection efficiency. Curr Gene Ther. 2008;8:313–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK084033/DK/NIDDK NIH HHS/United States
- 1R01AI07217699/AI/NIAID NIH HHS/United States
- U54 AR056953/AR/NIAMS NIH HHS/United States
- 1 U54 AR056953/AR/NIAMS NIH HHS/United States
- T32 GM007040/GM/NIGMS NIH HHS/United States
- R01 EY019555/EY/NEI NIH HHS/United States
- R01 AI080726/AI/NIAID NIH HHS/United States
- 5-P01-GM059299/GM/NIGMS NIH HHS/United States
- P01 GM059299/GM/NIGMS NIH HHS/United States
- 1R01EY01955518/EY/NEI NIH HHS/United States
- 1R01 AI0807268/AI/NIAID NIH HHS/United States
- P01 HL051818/HL/NHLBI NIH HHS/United States
- R01 AI072176/AI/NIAID NIH HHS/United States
- 2-P01-HL051818/HL/NHLBI NIH HHS/United States
- T32 AI007419/AI/NIAID NIH HHS/United States
- 1R01DK08403399/DK/NIDDK NIH HHS/United States
- 5T32-AI0074199/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

