Repeated reunions and splits feature the highly dynamic evolution of 5S and 35S ribosomal RNA genes (rDNA) in the Asteraceae family
- PMID: 20712858
- PMCID: PMC3095306
- DOI: 10.1186/1471-2229-10-176
Repeated reunions and splits feature the highly dynamic evolution of 5S and 35S ribosomal RNA genes (rDNA) in the Asteraceae family
Abstract
Background: In flowering plants and animals the most common ribosomal RNA genes (rDNA) organisation is that in which 35S (encoding 18S-5.8S-26S rRNA) and 5S genes are physically separated occupying different chromosomal loci. However, recent observations established that both genes have been unified to a single 35S-5S unit in the genus Artemisia (Asteraceae), a genomic arrangement typical of primitive eukaryotes such as yeast, among others. Here we aim to reveal the origin, distribution and mechanisms leading to the linked organisation of rDNA in the Asteraceae by analysing unit structure (PCR, Southern blot, sequencing), gene copy number (quantitative PCR) and chromosomal position (FISH) of 5S and 35S rRNA genes in approximately 200 species representing the family diversity and other closely related groups.
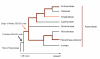
Results: Dominant linked rDNA genotype was found within three large groups in subfamily Asteroideae: tribe Anthemideae (93% of the studied cases), tribe Gnaphalieae (100%) and in the "Heliantheae alliance" (23%). The remaining five tribes of the Asteroideae displayed canonical non linked arrangement of rDNA, as did the other groups in the Asteraceae. Nevertheless, low copy linked genes were identified among several species that amplified unlinked units. The conserved position of functional 5S insertions downstream from the 26S gene suggests a unique, perhaps retrotransposon-mediated integration event at the base of subfamily Asteroideae. Further evolution likely involved divergence of 26S-5S intergenic spacers, amplification and homogenisation of units across the chromosomes and concomitant elimination of unlinked arrays. However, the opposite trend, from linked towards unlinked arrangement was also surmised in few species indicating possible reversibility of these processes.
Conclusions: Our results indicate that nearly 25% of Asteraceae species may have evolved unusual linked arrangement of rRNA genes. Thus, in plants, fundamental changes in intrinsic structure of rDNA units, their copy number and chromosomal organisation may occur within relatively short evolutionary time. We hypothesize that the 5S gene integration within the 35S unit might have repeatedly occurred during plant evolution, and probably once in Asteraceae.
Figures







Similar articles
-
Linkage of 35S and 5S rRNA genes in Artemisia (family Asteraceae): first evidence from angiosperms.Chromosoma. 2009 Feb;118(1):85-97. doi: 10.1007/s00412-008-0179-z. Epub 2008 Sep 9. Chromosoma. 2009. PMID: 18779974
-
Dancing together and separate again: gymnosperms exhibit frequent changes of fundamental 5S and 35S rRNA gene (rDNA) organisation.Heredity (Edinb). 2013 Jul;111(1):23-33. doi: 10.1038/hdy.2013.11. Epub 2013 Mar 20. Heredity (Edinb). 2013. PMID: 23512008 Free PMC article.
-
Cytogenetic features of rRNA genes across land plants: analysis of the Plant rDNA database.Plant J. 2017 Mar;89(5):1020-1030. doi: 10.1111/tpj.13442. Epub 2017 Feb 14. Plant J. 2017. PMID: 27943584
-
Ribosomal RNA genes in eukaryotic microorganisms: witnesses of phylogeny?FEMS Microbiol Rev. 2010 Jan;34(1):59-86. doi: 10.1111/j.1574-6976.2009.00196.x. FEMS Microbiol Rev. 2010. PMID: 19930463 Review.
-
5S rRNA gene arrangements in protists: a case of nonadaptive evolution.J Mol Evol. 2012 Jun;74(5-6):342-51. doi: 10.1007/s00239-012-9512-5. Epub 2012 Jul 11. J Mol Evol. 2012. PMID: 22782647 Review.
Cited by
-
Ribosomal Intergenic Spacers Are Filled with Transposon Remnants.Genome Biol Evol. 2023 Jul 3;15(7):evad114. doi: 10.1093/gbe/evad114. Genome Biol Evol. 2023. PMID: 37341531 Free PMC article.
-
Chromosomal diversification and karyotype evolution of diploids in the cytologically diverse genus Prospero (Hyacinthaceae).BMC Evol Biol. 2013 Jul 3;13:136. doi: 10.1186/1471-2148-13-136. BMC Evol Biol. 2013. PMID: 23819574 Free PMC article.
-
The striking and unexpected cytogenetic diversity of genus Tanacetum L. (Asteraceae): a cytometric and fluorescent in situ hybridisation study of Iranian taxa.BMC Plant Biol. 2015 Jul 8;15:174. doi: 10.1186/s12870-015-0564-8. BMC Plant Biol. 2015. PMID: 26152193 Free PMC article.
-
Genetic relationship study of some Vicia species by FISH and total seed storage protein patterns.J Genet Eng Biotechnol. 2020 Jul 31;18(1):37. doi: 10.1186/s43141-020-00054-6. J Genet Eng Biotechnol. 2020. PMID: 32737692 Free PMC article.
-
Retroelements and DNA Methylation Could Contribute to Diversity of 5S rDNA in Agave L.J Mol Evol. 2018 Jul;86(6):404-423. doi: 10.1007/s00239-018-9856-6. Epub 2018 Jul 10. J Mol Evol. 2018. PMID: 29992348
References
-
- Drouin G, de Sa MM. The concerted evolution of 5S ribosomal genes linked to the repeat units of other multigene families. Mol Biol Evol. 1995;12(3):481–493. - PubMed

