High mobility of bicoid captured by fluorescence correlation spectroscopy: implication for the rapid establishment of its gradient
- PMID: 20712981
- PMCID: PMC2920644
- DOI: 10.1016/j.bpj.2010.05.031
High mobility of bicoid captured by fluorescence correlation spectroscopy: implication for the rapid establishment of its gradient
Abstract
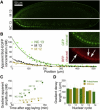
The Bicoid (Bcd) morphogen is essential for pattern formation in fruit flies. It forms an exponential concentration gradient along the embryo AP axis and turns on cascades of target genes in distinct anterior domains. The most commonly accepted model for gradient formation assumes that Bcd travels by simple diffusion and is uniformly degraded across syncytial embryos, yet several recent studies have challenged these ideas. Here, the question of Bcd mobility was investigated using fluorescence correlation spectroscopy in live Drosophila melanogaster embryos. Bcd-EGFP molecules were found to be highly mobile in the cytoplasm during cycles 12-14, with a diffusion coefficient approximately 7 microm(2)/s. This value is large enough to explain the stable establishment of the Bcd gradient simply by diffusion before cycle 8, i.e., before the onset of zygotic transcription.
2010 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Wolpert L. One hundred years of positional information. Trends Genet. 1996;12:359–364. - PubMed
-
- Crauk O., Dostatni N. Bicoid determines sharp and precise target gene expression in the Drosophila embryo. Curr. Biol. 2005;15:1888–1898. - PubMed
-
- Houchmandzadeh B., Wieschaus E., Leibler S. Precise domain specification in the developing Drosophila embryo. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2005;72:061920. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

