Rotating disk electrode voltammetric measurements of serotonin transporter kinetics in synaptosomes
- PMID: 20713085
- PMCID: PMC2952731
- DOI: 10.1016/j.jneumeth.2010.08.009
Rotating disk electrode voltammetric measurements of serotonin transporter kinetics in synaptosomes
Abstract
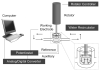
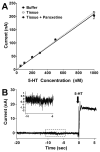
Altered serotonin (5-HT) signaling is implicated in several neuropsychiatric disorders, including depression, anxiety, obsessive-compulsive disorder, and autism. The 5-HT transporter (SERT) modulates 5-HT neurotransmission strength and duration. This is the first study using rotating disk electrode voltammetry (RDEV) to measure 5-HT clearance. SERT kinetics were measured in whole brain synaptosomes. Uptake kinetics of exogenous 5-HT were measured using glassy carbon electrodes rotated in 500 μL glass chambers containing synaptosomes from SERT-knockout (-/-), heterozygous (+/-), or wild-type (+/+) mice. RDEV detected 5-HT concentrations of 5nM and higher. Initial velocities were kinetically resolved with K(m) and V(max) values of 99±35 standard error of regression (SER) nM and 181±11 SER fmol/(s×mg protein), respectively in wild-type synaptosomes. The method enables control over drug and chemical concentrations, facilitating interpretation of results. Results are compared in detail to other techniques used to measure SERT kinetics, including tritium labeled assays, chronoamperometry, and fast scan cyclic voltammetry. RDEV exhibits decreased 5-HT detection limits, decreased vulnerability to 5-HT oxidation products that reduce electrode sensitivity, and also overcomes diffusion limitations via forced convection by providing a continuous, kinetically resolved signal. Finally, RDEV distinguishes functional differences between genotypes, notably, between wild-type and heterozygous mice, an experimental problem with other experimental approaches.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
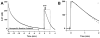
Similar articles
-
5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes.Synapse. 2012 Dec;66(12):1024-34. doi: 10.1002/syn.21608. Epub 2012 Sep 29. Synapse. 2012. PMID: 22961814 Free PMC article.
-
The contribution of low-affinity transport mechanisms to serotonin clearance in synaptosomes.Synapse. 2011 Oct;65(10):1015-23. doi: 10.1002/syn.20929. Epub 2011 Apr 7. Synapse. 2011. PMID: 21437992 Free PMC article.
-
Filtration disrupts synaptosomes during radiochemical analysis of serotonin uptake: comparison with chronoamperometry in SERT knockout mice.J Neurosci Methods. 2006 Jun 30;154(1-2):245-55. doi: 10.1016/j.jneumeth.2005.12.017. Epub 2006 Feb 10. J Neurosci Methods. 2006. PMID: 16472867
-
Serotonin Transporter Ala276 Mouse: Novel Model to Assess the Neurochemical and Behavioral Impact of Thr276 Phosphorylation In Vivo.Neurochem Res. 2022 Jan;47(1):37-60. doi: 10.1007/s11064-021-03299-w. Epub 2021 Apr 8. Neurochem Res. 2022. PMID: 33830406 Free PMC article. Review.
-
Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice.Neuropharmacology. 2008 Nov;55(6):1006-14. doi: 10.1016/j.neuropharm.2008.08.001. Epub 2008 Aug 12. Neuropharmacology. 2008. PMID: 18761360 Review.
Cited by
-
Effects of diet and insulin on dopamine transporter activity and expression in rat caudate-putamen, nucleus accumbens, and midbrain.J Neurochem. 2017 Mar;140(5):728-740. doi: 10.1111/jnc.13930. Epub 2017 Jan 31. J Neurochem. 2017. PMID: 27973691 Free PMC article.
-
Stress produces aversion and potentiates cocaine reward by releasing endogenous dynorphins in the ventral striatum to locally stimulate serotonin reuptake.J Neurosci. 2012 Dec 5;32(49):17582-96. doi: 10.1523/JNEUROSCI.3220-12.2012. J Neurosci. 2012. PMID: 23223282 Free PMC article.
-
5-HT(1B) autoreceptor regulation of serotonin transporter activity in synaptosomes.Synapse. 2012 Dec;66(12):1024-34. doi: 10.1002/syn.21608. Epub 2012 Sep 29. Synapse. 2012. PMID: 22961814 Free PMC article.
-
Molecular fMRI of Serotonin Transport.Neuron. 2016 Nov 23;92(4):754-765. doi: 10.1016/j.neuron.2016.09.048. Epub 2016 Oct 20. Neuron. 2016. PMID: 27773583 Free PMC article.
-
The contribution of low-affinity transport mechanisms to serotonin clearance in synaptosomes.Synapse. 2011 Oct;65(10):1015-23. doi: 10.1002/syn.20929. Epub 2011 Apr 7. Synapse. 2011. PMID: 21437992 Free PMC article.
References
-
- Amara SG, Sonders MS. Neurotransmitter transporters as molecular targets for addictive drugs. Drug Alcohol Depend. 1998;51:87–96. - PubMed
-
- Ansah TA, Ramamoorthy S, Montanez S, Daws LC, Blakely RD. Calcium-dependent inhibition of synaptosomal serotonin transport by the alpha 2-adrenoceptor agonist 5-bromo-N-[4,5-dihydro-1H-imidazol-2-yl]-6-quinoxalinamine (UK14304) J Pharmacol Exp Ther. 2003;305:956–65. - PubMed
-
- Asano S, Matsuda T, Nakasu Y, Maeda S, Nogi H, Baba A. Inhibition by nitric oxide of the uptake of [3H]serotonin into rat brain synaptosomes. Jpn J Pharmacol. 1997;75:123–8. - PubMed
-
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (“Ecstasy”) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

