Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo
- PMID: 20713173
- PMCID: PMC2950250
- DOI: 10.1016/j.biocel.2010.08.004
Adjudin-mediated Sertoli-germ cell junction disassembly affects Sertoli cell barrier function in vitro and in vivo
Abstract
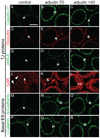
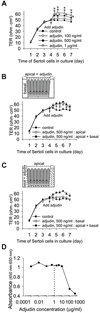
Adjudin, an analogue of lonidamine, affects adhesion between Sertoli and most germ cells, resulting in reversible infertility in rats, rabbits and dogs. Previous studies have described the apical ectoplasmic specialization, a hybrid-type of Sertoli cell-elongating/elongated spermatid adhesive junction, as a key target of adjudin. In this study, we ask if the function of the blood-testis barrier which is constituted by co-existing tight junctions, desmosome-gap junctions and basal ectoplasmic specializations can be maintained when the seminiferous epithelium is under assault by adjudin. We report herein that administration of a single oral dose of adjudin to adult rats increased the levels of several tight junction and basal ectoplasmic specialization proteins during germ cell loss from the seminiferous epithelium. These findings were corroborated by a functional in vitro experiment when Sertoli cells were cultured on Matrigel™-coated bicameral units in the presence of adjudin and transepithelial electrical resistance was quantified across the epithelium. Indeed, the Sertoli cell permeability barrier was shown to become tighter after adjudin treatment as evidenced by an increase in transepithelial electrical resistance. Equally important, the blood-testis barrier in adjudin-treated rats was shown to be intact 2 weeks post-treatment when its integrity was monitored following vascular administration of inulin-fluorescein isothiocyanate which failed to permeate past the barrier and enter into the adluminal compartment. These results illustrate that a unique mechanism exists to maintain blood-testis barrier integrity at all costs, irrespective of the presence of germ cells in the seminiferous epithelium of the testis.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures





References
-
- Baillie AH, Griffiths K. 3β-Hydroxysteroid dehydrogenase activity in the mouse Leydig cell. J Endocrinol. 1964;29:9–17. - PubMed
-
- Balda MS, Matter K. Tight junctions and the regulation of gene expression. Biochim Biophys Acta. 2009;1788:761–767. - PubMed
-
- Beaver BV, Reed W, Leary S, McKiernan B, Bain F, Schultz R, et al. 2000 Report of the American Veterinary Medical Association Panel in Euthanasia. J Am Vet Med Assoc. 2001;218:669–696. - PubMed
-
- Ben-Ze'ev A, Geiger B. Differential molecular interactions of β-catenin and plakoglobin in adhesion, signaling, and cancer. Curr Opin Cell Biol. 1998;10:629–639. - PubMed
-
- Byers S, Hadley MA, Djakiew D, Dym M. Growth and characterization of epididymal epithelial cells and Sertoli cells in dual environment culture chambers. J Androl. 1986;7:59–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

