Role of ROS signaling in differential hypoxic Ca2+ and contractile responses in pulmonary and systemic vascular smooth muscle cells
- PMID: 20713188
- PMCID: PMC2991600
- DOI: 10.1016/j.resp.2010.08.008
Role of ROS signaling in differential hypoxic Ca2+ and contractile responses in pulmonary and systemic vascular smooth muscle cells
Abstract
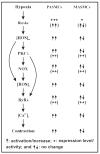
Hypoxia causes a large increase in [Ca2+]i and attendant contraction in pulmonary artery smooth muscle cells (PASMCs), but not in systemic artery SMCs. The different responses meet the respective functional needs in these two distinct vascular myocytes; however, the underlying molecular mechanisms are not well known. We and other investigators have provided extensive evidence to reveal that voltage-dependent K+ (KV) channels, canonical transient receptor potential (TRPC) channels, ryanodine receptor Ca2+ release channels (RyRs), cyclic adenosine diphosphate-ribose, FK506 binding protein 12.6, protein kinase C, NADPH oxidase and reactive oxygen species (ROS) are the essential effectors and signaling intermediates in the hypoxic increase in [Ca2+]i in PASMCs and HPV, but they may not primarily underlie the diverse cellular responses in pulmonary and systemic vascular myocytes. Hypoxia significantly increases mitochondrial ROS generation in PASMCs, which can induce intracellular Ca2+ release by opening RyRs, and may also cause extracellular Ca2+ influx by inhibiting KV channels and activating TRPC channels, leading to a large increase in [Ca2+]i in PASMCs and HPV. In contrast, hypoxia has no or a minor effect on mitochondrial ROS generation in systemic SMCs, thereby causing no change or a negligible increase in [Ca2+]i and contraction. Further preliminary work indicates that Rieske iron-sulfur protein in the mitochondrial complex III may perhaps serve as a key initial molecular determinant for the hypoxic increase in [Ca2+]i in PASMCs and HPV, suggesting its potential important role in different cellular changes to respond to hypoxic stimulation in pulmonary and systemic artery myocytes. All these findings have greatly improved our understanding of the molecular processes for the differential hypoxic Ca2+ and contractile responses in vascular SMCs from distinct pulmonary and systemic circulation systems.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
ROS-dependent signaling mechanisms for hypoxic Ca(2+) responses in pulmonary artery myocytes.Antioxid Redox Signal. 2010 Mar 1;12(5):611-23. doi: 10.1089/ars.2009.2877. Antioxid Redox Signal. 2010. PMID: 19764882 Free PMC article. Review.
-
Endoplasmic reticulum Ca2+ release causes Rieske iron-sulfur protein-mediated mitochondrial ROS generation in pulmonary artery smooth muscle cells.Biosci Rep. 2019 Dec 20;39(12):BSR20192414. doi: 10.1042/BSR20192414. Biosci Rep. 2019. Retraction in: Biosci Rep. 2020 Apr 30;40(4):BSR-20192414_RET. doi: 10.1042/BSR-20192414_RET. PMID: 31710081 Free PMC article. Retracted.
-
Type-3 ryanodine receptors mediate hypoxia-, but not neurotransmitter-induced calcium release and contraction in pulmonary artery smooth muscle cells.J Gen Physiol. 2005 Apr;125(4):427-40. doi: 10.1085/jgp.200409232. J Gen Physiol. 2005. PMID: 15795312 Free PMC article.
-
Important Role of Sarcoplasmic Reticulum Ca2+ Release via Ryanodine Receptor-2 Channel in Hypoxia-Induced Rieske Iron-Sulfur Protein-Mediated Mitochondrial Reactive Oxygen Species Generation in Pulmonary Artery Smooth Muscle Cells.Antioxid Redox Signal. 2020 Mar 1;32(7):447-462. doi: 10.1089/ars.2018.7652. Epub 2019 Oct 11. Antioxid Redox Signal. 2020. PMID: 31456413 Free PMC article.
-
Oxygen sensing and signal transduction in hypoxic pulmonary vasoconstriction.Eur Respir J. 2016 Jan;47(1):288-303. doi: 10.1183/13993003.00945-2015. Epub 2015 Oct 22. Eur Respir J. 2016. PMID: 26493804 Review.
Cited by
-
The Xanthine Derivative KMUP-1 Inhibits Hypoxia-Induced TRPC1 Expression and Store-Operated Ca2+ Entry in Pulmonary Arterial Smooth Muscle Cells.Pharmaceuticals (Basel). 2024 Mar 29;17(4):440. doi: 10.3390/ph17040440. Pharmaceuticals (Basel). 2024. PMID: 38675401 Free PMC article.
-
O2 sensing, mitochondria and ROS signaling: The fog is lifting.Mol Aspects Med. 2016 Feb-Mar;47-48:76-89. doi: 10.1016/j.mam.2016.01.002. Epub 2016 Jan 14. Mol Aspects Med. 2016. PMID: 26776678 Free PMC article. Review.
-
"Oxygen Sensing" by Na,K-ATPase: These Miraculous Thiols.Front Physiol. 2016 Aug 2;7:314. doi: 10.3389/fphys.2016.00314. eCollection 2016. Front Physiol. 2016. PMID: 27531981 Free PMC article. Review.
-
Sulfhydryl-dependent dimerization of soluble guanylyl cyclase modulates the relaxation of porcine pulmonary arteries to nitric oxide.Pflugers Arch. 2013 Feb;465(2):333-41. doi: 10.1007/s00424-012-1176-x. Epub 2012 Nov 10. Pflugers Arch. 2013. PMID: 23143201
-
Elevated Perspectives: Unraveling Cardiovascular Dynamics in High-Altitude Realms.Curr Cardiol Rev. 2025;21(2):e1573403X308818. doi: 10.2174/011573403X308818241030051249. Curr Cardiol Rev. 2025. PMID: 39506447 Review.
References
-
- Archer SL, Huang J, Henry T, Peterson D, Weir EK. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. - PubMed
-
- Archer SL, Huang JM, Reeve HL, Hampl V, Tolarova S, Michelakis E, Weir EK. Differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

