Cost-effectiveness of using human papillomavirus 16/18 genotype triage in cervical cancer screening
- PMID: 20713299
- PMCID: PMC4568837
- DOI: 10.1016/j.ygyno.2010.07.004
Cost-effectiveness of using human papillomavirus 16/18 genotype triage in cervical cancer screening
Abstract
Objective: Testing for human papillomavirus (HPV) 16 and 18 genotypes, which are known to cause approximately 65-70% of invasive cervical cancer cases, may allow clinicians to identify women at highest risk for underlying cervical intraepithelial neoplasia missed by Pap cytology. Our objective was to determine the cost-effectiveness of adding HPV-16 and 18 genotype triage to current cervical cancer screening strategies in the United States.
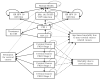
Methods: We developed a lifetime Markov model to assess the cost-effectiveness of the following cervical cancer screening algorithms: (1) liquid-based cytology (LBC), (2) LBC+HPV triage, (3) HPV+LBC triage, (4) co-screening, (5) co-screening+HPV genotyping, and (6) HPV only+HPV genotyping. Costs were estimated from a payer perspective in 2007 U.S. dollars. Outcome measures included lifetime risk of cervical cancer, quality-adjusted life years saved (QALYs), and incremental cost-effectiveness ratios (ICERs).
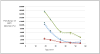
Results: In our model, the use of HPV genotyping strategies prevented 51-73 deaths per 100,000 women screened compared to screening using LBC followed by HPV triage and 4-26 deaths compared to co-screening with LBC and high-risk HPV. Use of HPV genotyping to triage all high-risk HPV-positive women every three years had an ICER of $34,074 per QALY compared to HPV and LBC co-screening. HPV genotyping with co-screening was the most effective strategy and had an ICER of $33,807 per QALY compared to HPV genotyping for all high-risk HPV-positive women.
Conclusion: The addition of HPV-16 and -18 genotype triage to HPV and LBC co-screening was a cost-effective screening strategy in the United States.
Copyright © 2010. Published by Elsevier Inc.
Figures

Similar articles
-
Comparison of HPV-16 and HPV-18 Genotyping and Cytological Testing as Triage Testing Within Human Papillomavirus-Based Screening in Mexico.JAMA Netw Open. 2019 Nov 1;2(11):e1915781. doi: 10.1001/jamanetworkopen.2019.15781. JAMA Netw Open. 2019. PMID: 31747033 Free PMC article.
-
Detection Rate of High-Grade Cervical Neoplasia and Cost-Effectiveness of High-Risk Human Papillomavirus Genotyping with Reflex Liquid-based Cytology in Cervical Cancer Screening.Ann Acad Med Singap. 2017 Jul;46(7):267-273. Ann Acad Med Singap. 2017. PMID: 28821890
-
The clinical effectiveness and cost-effectiveness of primary human papillomavirus cervical screening in England: extended follow-up of the ARTISTIC randomised trial cohort through three screening rounds.Health Technol Assess. 2014 Apr;18(23):1-196. doi: 10.3310/hta18230. Health Technol Assess. 2014. PMID: 24762804 Free PMC article. Clinical Trial.
-
[Cost-effectiveness analysis of cervical cancer screening strategies in urban China].Zhonghua Zhong Liu Za Zhi. 2019 Feb 23;41(2):154-160. doi: 10.3760/cma.j.issn.0253-3766.2019.02.015. Zhonghua Zhong Liu Za Zhi. 2019. PMID: 30862148 Review. Chinese.
-
Prophylaxis of cervical cancer and related cervical disease: a review of the cost-effectiveness of vaccination against oncogenic HPV types.J Manag Care Pharm. 2010 Apr;16(3):217-30. doi: 10.18553/jmcp.2010.16.3.217. J Manag Care Pharm. 2010. PMID: 20331326 Free PMC article. Review.
Cited by
-
The Next Generation of Cervical Cancer Screening: Should Guidelines Focus on Best Practices for the Future or Current Screening Capacity?J Low Genit Tract Dis. 2018 Apr;22(2):91-96. doi: 10.1097/LGT.0000000000000378. J Low Genit Tract Dis. 2018. PMID: 29570563 Free PMC article. No abstract available.
-
Systematic review of model-based cervical screening evaluations.BMC Cancer. 2015 May 1;15:334. doi: 10.1186/s12885-015-1332-8. BMC Cancer. 2015. PMID: 25924871 Free PMC article.
-
Estimated Quality of Life and Economic Outcomes Associated With 12 Cervical Cancer Screening Strategies: A Cost-effectiveness Analysis.JAMA Intern Med. 2019 Jul 1;179(7):867-878. doi: 10.1001/jamainternmed.2019.0299. JAMA Intern Med. 2019. PMID: 31081851 Free PMC article.
-
The Clinical and Economic Benefits of Co-Testing Versus Primary HPV Testing for Cervical Cancer Screening: A Modeling Analysis.J Womens Health (Larchmt). 2016 Jun;25(6):606-16. doi: 10.1089/jwh.2015.5708. Epub 2016 Mar 29. J Womens Health (Larchmt). 2016. PMID: 27023044 Free PMC article.
-
Cost effectiveness of human papillomavirus-16/18 genotyping in cervical cancer screening.Appl Health Econ Health Policy. 2015 Feb;13(1):95-107. doi: 10.1007/s40258-014-0135-4. Appl Health Econ Health Policy. 2015. PMID: 25385310 Free PMC article.
References
-
- Munoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. for the International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–27. - PubMed
-
- Cuzick J, Arbyn M, Sankaranarayanan R, Tsu V, Ronco G, Mayrand MH, et al. Overview of human papillomavirus-based and other novel options for cervical cancer screening in developed and developing countries. Vaccine. 2008;26S:K29–41. - PubMed

