Treatment monitoring of paranasal sinus tumors by magnetic resonance imaging
- PMID: 20713318
- PMCID: PMC2943677
- DOI: 10.1102/1470-7330.2010.0025
Treatment monitoring of paranasal sinus tumors by magnetic resonance imaging
Abstract
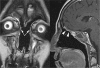
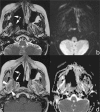
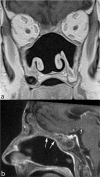
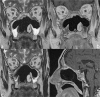
Treatment monitoring of paranasal tumors is crucial, given the high rate of local and regional relapses that impairs the overall prognosis of patients. Magnetic resonance imaging (MRI) is the technique of choice to detect changes in the submucosa and deep spaces of the suprahyoid neck, inaccessible at clinical and endoscopic assessment. Correct interpretation of MRI requires detailed knowledge of the treatment applied and of the changes treatments are supposed to produce on macroscopic anatomy and tissue signals. Once such background of information is obtained, detection of recurrences is a less challenging task.
Figures

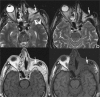

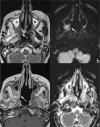
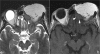

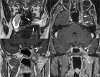
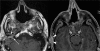
References
-
- Khademi B, Moradi A, Hoseini S, Mohammadianpanah M. Malignant neoplasms of the sinonasal tract: report of 71 patients and literature review and analysis. Oral Maxillofac Surg. 2009;13:191–9. doi:10.1007/s10006-009-0170-8. PMid:19795137. - DOI - PubMed
-
- Mendenhall WM, Mendenhall CM, Riggs CE, Jr, Villaret DB, Mendenhall NP. Sinonasal undifferentiated carcinoma. Am J Clin Oncol. 2006;29:27–31. doi:10.1097/01.coc.0000189691.04140.02. PMid:16462499. - DOI - PubMed
-
- Dulguerov P, Jacobsen MS, Allal AS, Lehmann W, Calcaterra T. Nasal and paranasal sinus carcinoma: are we making progress? A series of 220 patients and a systematic review. Cancer. 2001;92:3012–29. doi:10.1002/1097-0142(20011215)92:12<3012::AID-CNCR10131>3.0.CO;2-E. - DOI - PubMed
-
- Dulguerov P, Allal AS. Nasal and paranasal sinus carcinoma: how can we continue to make progress? Curr Opin Otolaryngol Head Neck Surg. 2006;14:67–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
