Srs2 plays a critical role in reversible G2 arrest upon chronic and low doses of UV irradiation via two distinct homologous recombination-dependent mechanisms in postreplication repair-deficient cells
- PMID: 20713444
- PMCID: PMC2950541
- DOI: 10.1128/MCB.00453-10
Srs2 plays a critical role in reversible G2 arrest upon chronic and low doses of UV irradiation via two distinct homologous recombination-dependent mechanisms in postreplication repair-deficient cells
Abstract
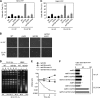
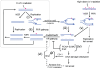
Differential posttranslational modification of proliferating cell nuclear antigen (PCNA) by ubiquitin or SUMO plays an important role in coordinating the processes of DNA replication and DNA damage tolerance. Previously it was shown that the loss of RAD6-dependent error-free postreplication repair (PRR) results in DNA damage checkpoint-mediated G(2) arrest in cells exposed to chronic low-dose UV radiation (CLUV), whereas wild-type and nucleotide excision repair-deficient cells are largely unaffected. In this study, we report that suppression of homologous recombination (HR) in PRR-deficient cells by Srs2 and PCNA sumoylation is required for checkpoint activation and checkpoint maintenance during CLUV irradiation. Cyclin-dependent kinase (CDK1)-dependent phosphorylation of Srs2 did not influence checkpoint-mediated G(2) arrest or maintenance in PRR-deficient cells but was critical for HR-dependent checkpoint recovery following release from CLUV exposure. These results indicate that Srs2 plays an important role in checkpoint-mediated reversible G(2) arrest in PRR-deficient cells via two separate HR-dependent mechanisms. The first (required to suppress HR during PRR) is regulated by PCNA sumoylation, whereas the second (required for HR-dependent recovery following CLUV exposure) is regulated by CDK1-dependent phosphorylation.
Figures
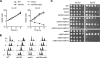

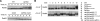


Similar articles
-
RAD6-RAD18-RAD5-pathway-dependent tolerance to chronic low-dose ultraviolet light.Nature. 2009 Jan 29;457(7229):612-5. doi: 10.1038/nature07580. Epub 2008 Dec 14. Nature. 2009. PMID: 19079240
-
Functional and physical interaction of yeast Mgs1 with PCNA: impact on RAD6-dependent DNA damage tolerance.Mol Cell Biol. 2006 Jul;26(14):5509-17. doi: 10.1128/MCB.00307-06. Mol Cell Biol. 2006. PMID: 16809783 Free PMC article.
-
The Saccharomyces cerevisiae Rad6 postreplication repair and Siz1/Srs2 homologous recombination-inhibiting pathways process DNA damage that arises in asf1 mutants.Mol Cell Biol. 2009 Oct;29(19):5226-37. doi: 10.1128/MCB.00894-09. Epub 2009 Jul 27. Mol Cell Biol. 2009. PMID: 19635810 Free PMC article.
-
Error-free DNA-damage tolerance in Saccharomyces cerevisiae.Mutat Res Rev Mutat Res. 2015 Apr-Jun;764:43-50. doi: 10.1016/j.mrrev.2015.02.001. Epub 2015 Feb 16. Mutat Res Rev Mutat Res. 2015. PMID: 26041265 Review.
-
Multifunctional roles of Saccharomyces cerevisiae Srs2 protein in replication, recombination and repair.FEMS Yeast Res. 2017 Mar 1;17(2):fow111. doi: 10.1093/femsyr/fow111. FEMS Yeast Res. 2017. PMID: 28011904 Free PMC article. Review.
Cited by
-
Genetic instability in budding and fission yeast-sources and mechanisms.FEMS Microbiol Rev. 2015 Nov;39(6):917-67. doi: 10.1093/femsre/fuv028. Epub 2015 Jun 24. FEMS Microbiol Rev. 2015. PMID: 26109598 Free PMC article. Review.
-
Shared genetic pathways contribute to the tolerance of endogenous and low-dose exogenous DNA damage in yeast.Genetics. 2014 Oct;198(2):519-30. doi: 10.1534/genetics.114.168617. Epub 2014 Jul 24. Genetics. 2014. PMID: 25060101 Free PMC article.
-
Functions of Fun30 chromatin remodeler in regulating cellular resistance to genotoxic stress.PLoS One. 2015 Mar 25;10(3):e0121341. doi: 10.1371/journal.pone.0121341. eCollection 2015. PLoS One. 2015. PMID: 25806814 Free PMC article.
-
Chronic low-dose ultraviolet-induced mutagenesis in nucleotide excision repair-deficient cells.Nucleic Acids Res. 2012 Sep 1;40(17):8406-15. doi: 10.1093/nar/gks580. Epub 2012 Jun 28. Nucleic Acids Res. 2012. PMID: 22743272 Free PMC article.
-
DNA Damage Tolerance Pathway Choice Through Uls1 Modulation of Srs2 SUMOylation in Saccharomyces cerevisiae.Genetics. 2017 May;206(1):513-525. doi: 10.1534/genetics.116.196568. Epub 2017 Mar 24. Genetics. 2017. PMID: 28341648 Free PMC article.
References
-
- Amberg, D. C., D. J. Burke, and J. N. Strathern. 2005. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Andersen, P. L., F. Xu, and W. Xiao. 2008. Eukaryotic DNA damage tolerance and translesion synthesis through covalent modifications of PCNA. Cell Res. 18:162-173. - PubMed
-
- Bartkova, J., Z. Horejsi, K. Koed, A. Kramer, F. Tort, K. Zieger, P. Guldberg, M. Sehested, J. M. Nesland, C. Lukas, T. Orntoft, J. Lukas, and J. Bartek. 2005. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature 434:864-870. - PubMed
-
- Bergink, S., and S. Jentsch. 2009. Principles of ubiquitin and SUMO modifications in DNA repair. Nature 458:461-467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
