Induction of nonapoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6
- PMID: 20713492
- PMCID: PMC2994602
- DOI: 10.1158/1541-7786.MCR-10-0090
Induction of nonapoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6
Abstract
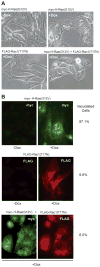
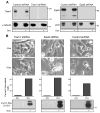
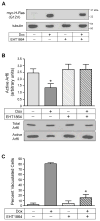
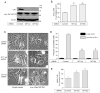
Methuosis is a unique form of nonapoptotic cell death triggered by alterations in the trafficking of clathrin-independent endosomes, ultimately leading to extreme vacuolization and rupture of the cell. Methuosis can be induced in glioblastoma cells by expression of constitutively active Ras. This study identifies the small GTPases, Rac1 and Arf6, and the Arf6 GTPase-activating protein, GIT1, as key downstream components of the signaling pathway underlying Ras-induced methuosis. The extent to which graded expression of active H-Ras(G12V) triggers cytoplasmic vacuolization correlates with the amount of endogenous Rac1 in the active GTP state. Blocking Rac1 activation with the specific Rac inhibitor, EHT 1864, or coexpression of dominant-negative Rac1(T17N), prevents the accumulation of vacuoles induced by H-Ras(G12V). Coincident with Rac1 activation, H-Ras(G12V) causes a decrease in the amount of active Arf6, a GTPase that functions in the recycling of clathrin-independent endosomes. The effect of H-Ras(G12V) on Arf6 is blocked by EHT 1864, indicating that the decrease in Arf6-GTP is directly linked to the activation of Rac1. Constitutively active Rac1(G12V) interacts with GIT1 in immunoprecipitation assays. Ablation of GIT1 by short hairpin RNA prevents the decrease in active Arf6, inhibits vacuolization, and prevents loss of cell viability in cells expressing Rac1(G12V). Together, the results suggest that perturbations of endosome morphology associated with Ras-induced methuosis are due to downstream activation of Rac1 combined with reciprocal inactivation of Arf6. The latter seems to be mediated through Rac1 stimulation of GIT1. Further insights into this pathway could suggest opportunities for the induction of methuosis in cancers that are resistant to apoptotic cell death.
Figures





Similar articles
-
A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells.Mol Cancer. 2011 Jun 6;10:69. doi: 10.1186/1476-4598-10-69. Mol Cancer. 2011. PMID: 21639944 Free PMC article.
-
ARF6-Rac1 signaling-mediated neurite outgrowth is potentiated by the neuronal adaptor FE65 through orchestrating ARF6 and ELMO1.FASEB J. 2020 Dec;34(12):16397-16413. doi: 10.1096/fj.202001703R. Epub 2020 Oct 13. FASEB J. 2020. PMID: 33047393
-
Methuosis: nonapoptotic cell death associated with vacuolization of macropinosome and endosome compartments.Am J Pathol. 2014 Jun;184(6):1630-42. doi: 10.1016/j.ajpath.2014.02.028. Epub 2014 Apr 13. Am J Pathol. 2014. PMID: 24726643 Free PMC article. Review.
-
ADP-ribosylation factor 6 regulates glioma cell invasion through the IQ-domain GTPase-activating protein 1-Rac1-mediated pathway.Cancer Res. 2009 Feb 1;69(3):794-801. doi: 10.1158/0008-5472.CAN-08-2110. Epub 2009 Jan 20. Cancer Res. 2009. PMID: 19155310 Free PMC article.
-
GTPases Rac1 and Ras Signaling from Endosomes.Prog Mol Subcell Biol. 2018;57:65-105. doi: 10.1007/978-3-319-96704-2_3. Prog Mol Subcell Biol. 2018. PMID: 30097772 Review.
Cited by
-
Cytoplasmic vacuolization in cell death and survival.Oncotarget. 2016 Aug 23;7(34):55863-55889. doi: 10.18632/oncotarget.10150. Oncotarget. 2016. PMID: 27331412 Free PMC article. Review.
-
Protease 3C of hepatitis A virus induces vacuolization of lysosomal/endosomal organelles and caspase-independent cell death.BMC Cell Biol. 2015 Feb 27;16:4. doi: 10.1186/s12860-015-0050-z. BMC Cell Biol. 2015. PMID: 25886889 Free PMC article.
-
miR-199a-3p displays tumor suppressor functions in papillary thyroid carcinoma.Oncotarget. 2014 May 15;5(9):2513-28. doi: 10.18632/oncotarget.1830. Oncotarget. 2014. PMID: 24810336 Free PMC article.
-
A chalcone-related small molecule that induces methuosis, a novel form of non-apoptotic cell death, in glioblastoma cells.Mol Cancer. 2011 Jun 6;10:69. doi: 10.1186/1476-4598-10-69. Mol Cancer. 2011. PMID: 21639944 Free PMC article.
-
Cellular Regulation of Macropinocytosis.Int J Mol Sci. 2024 Jun 26;25(13):6963. doi: 10.3390/ijms25136963. Int J Mol Sci. 2024. PMID: 39000072 Free PMC article. Review.
References
-
- Bursch W, Ellinger A, Gerner C, Frohwein U, Schulte-Hermann R. Programmed cell death (PCD). Apoptosis, autophagic PCD, or others? Ann N Y Acad Sci. 2000;926:1–12. - PubMed
-
- Lockshin RA, Zakeri Z. Apoptosis, autophagy, and more. Int J Biochem Cell Biol. 2004;36:2405–2419. - PubMed
-
- Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. - PubMed
-
- Wang Y, Li X, Wang L, Ding P, Zhang Y, Han W, Ma D. An alternative form of paraptosis-like cell death, triggered by TAJ/TROY and enhanced by PDCD5 overexpression. J Cell Sci. 2004;117:1525–1532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

