Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions
- PMID: 20713515
- PMCID: PMC2922499
- DOI: 10.1101/gad.1953910
Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions
Abstract
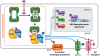
The plant hormone abscisic acid (ABA) regulates many key processes in plants, including seed germination and development and abiotic stress tolerance, particularly drought resistance. Understanding early events in ABA signal transduction has been a major goal of plant research. The recent identification of the PYRABACTIN (4-bromo-N-[pyridin-2-yl methyl]naphthalene-1-sulfonamide) RESISTANCE (PYR)/REGULATORY COMPONENT OF ABA RECEPTOR (RCAR) family of ABA receptors and their biochemical mode of action represents a major breakthrough in the field. The solving of PYR/RCAR structures provides a context for resolving mechanisms mediating ABA control of protein-protein interactions for downstream signaling. Recent studies show that a pathway based on PYR/RCAR ABA receptors, PROTEIN PHOSPHATASE 2Cs (PP2Cs), and SNF1-RELATED PROTEIN KINASE 2s (SnRK2s) forms the primary basis of an early ABA signaling module. This pathway interfaces with ion channels, transcription factors, and other targets, thus providing a mechanistic connection between the phytohormone and ABA-induced responses. This emerging PYR/RCAR-PP2C-SnRK2 model of ABA signal transduction is reviewed here, and provides an opportunity for testing novel hypotheses concerning ABA signaling. We address newly emerging questions, including the potential roles of different PYR/RCAR isoforms, and the significance of ABA-induced versus constitutive PYR/RCAR-PP2C interactions. We also consider how the PYR/RCAR-PP2C-SnRK2 pathway interfaces with ABA-dependent gene expression, ion channel regulation, and control of small molecule signaling. These exciting developments provide researchers with a framework through which early ABA signaling can be understood, and allow novel questions about the hormone response pathway and possible applications in stress resistance engineering of plants to be addressed.
Figures
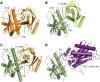

References
-
- Albrecht V, Weinl S, Blazevic D, D'Angelo C, Batistic O, Kolukisaoglu Ü, Bock R, Schulz B, Harter K, Kudla J 2003. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J 36: 457–470 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
