Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells
- PMID: 20713546
- PMCID: PMC2931145
- DOI: 10.1085/jgp.201010453
Type 1 IP3 receptors activate BKCa channels via local molecular coupling in arterial smooth muscle cells
Abstract
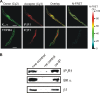
Plasma membrane large-conductance Ca(2+)-activated K(+) (BK(Ca)) channels and sarcoplasmic reticulum inositol 1,4,5-trisphosphate (IP(3)) receptors (IP(3)Rs) are expressed in a wide variety of cell types, including arterial smooth muscle cells. Here, we studied BK(Ca) channel regulation by IP(3) and IP(3)Rs in rat and mouse cerebral artery smooth muscle cells. IP(3) activated BK(Ca) channels both in intact cells and in excised inside-out membrane patches. IP(3) caused concentration-dependent BK(Ca) channel activation with an apparent dissociation constant (K(d)) of approximately 4 microM at physiological voltage (-40 mV) and intracellular Ca(2+) concentration ([Ca(2+)](i); 10 microM). IP(3) also caused a leftward-shift in BK(Ca) channel apparent Ca(2+) sensitivity and reduced the K(d) for free [Ca(2+)](i) from approximately 20 to 12 microM, but did not alter the slope or maximal P(o). BAPTA, a fast Ca(2+) buffer, or an elevation in extracellular Ca(2+) concentration did not alter IP(3)-induced BK(Ca) channel activation. Heparin, an IP(3)R inhibitor, and a monoclonal type 1 IP(3)R (IP(3)R1) antibody blocked IP(3)-induced BK(Ca) channel activation. Adenophostin A, an IP(3)R agonist, also activated BK(Ca) channels. IP(3) activated BK(Ca) channels in inside-out patches from wild-type (IP(3)R1(+/+)) mouse arterial smooth muscle cells, but had no effect on BK(Ca) channels of IP(3)R1-deficient (IP(3)R1(-/-)) mice. Immunofluorescence resonance energy transfer microscopy indicated that IP(3)R1 is located in close spatial proximity to BK(Ca) alpha subunits. The IP(3)R1 monoclonal antibody coimmunoprecipitated IP(3)R1 and BK(Ca) channel alpha and beta1 subunits from cerebral arteries. In summary, data indicate that IP(3)R1 activation elevates BK(Ca) channel apparent Ca(2+) sensitivity through local molecular coupling in arterial smooth muscle cells.
Figures
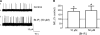
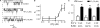



Comment in
-
Interaction between IP₃ receptors and BK channels in arterial smooth muscle: non-canonical IP₃ signaling at work.J Gen Physiol. 2011 May;137(5):473-7. doi: 10.1085/jgp.201110607. Epub 2011 Apr 11. J Gen Physiol. 2011. PMID: 21482693 Free PMC article. No abstract available.
Similar articles
-
IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release.Circ Res. 2008 May 9;102(9):1118-26. doi: 10.1161/CIRCRESAHA.108.173948. Epub 2008 Apr 3. Circ Res. 2008. PMID: 18388325 Free PMC article.
-
LRRC26 is a functional BK channel auxiliary γ subunit in arterial smooth muscle cells.Circ Res. 2014 Aug 1;115(4):423-31. doi: 10.1161/CIRCRESAHA.115.303407. Epub 2014 Jun 6. Circ Res. 2014. PMID: 24906643 Free PMC article.
-
Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle.Am J Physiol Cell Physiol. 2001 Dec;281(6):C1769-75. doi: 10.1152/ajpcell.2001.281.6.C1769. Am J Physiol Cell Physiol. 2001. PMID: 11698234
-
The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease.Pflugers Arch. 2008 Aug;456(5):769-85. doi: 10.1007/s00424-008-0491-8. Epub 2008 Mar 26. Pflugers Arch. 2008. PMID: 18365243 Free PMC article. Review.
-
Function and regulation of large conductance Ca(2+)-activated K+ channel in vascular smooth muscle cells.Drug Discov Today. 2012 Sep;17(17-18):974-87. doi: 10.1016/j.drudis.2012.04.002. Epub 2012 Apr 13. Drug Discov Today. 2012. PMID: 22521666 Free PMC article. Review.
Cited by
-
Angiotensin II stimulates internalization and degradation of arterial myocyte plasma membrane BK channels to induce vasoconstriction.Am J Physiol Cell Physiol. 2015 Sep 15;309(6):C392-402. doi: 10.1152/ajpcell.00127.2015. Epub 2015 Jul 15. Am J Physiol Cell Physiol. 2015. PMID: 26179602 Free PMC article.
-
Changes in IP3 Receptor Expression and Function in Aortic Smooth Muscle of Atherosclerotic Mice.J Vasc Res. 2017;54(2):68-78. doi: 10.1159/000461581. Epub 2017 Apr 1. J Vasc Res. 2017. PMID: 28365690 Free PMC article.
-
STIM1 enhances SR Ca2+ content through binding phospholamban in rat ventricular myocytes.Proc Natl Acad Sci U S A. 2015 Aug 25;112(34):E4792-801. doi: 10.1073/pnas.1423295112. Epub 2015 Aug 10. Proc Natl Acad Sci U S A. 2015. PMID: 26261328 Free PMC article.
-
Inositol 1,4,5-trisphosphate receptors and their protein partners as signalling hubs.J Physiol. 2016 Jun 1;594(11):2849-66. doi: 10.1113/JP271139. Epub 2016 Feb 24. J Physiol. 2016. PMID: 26830355 Free PMC article. Review.
-
A Chlorzoxazone-Baclofen Combination Improves Cerebellar Impairment in Spinocerebellar Ataxia Type 1.Mov Disord. 2021 Mar;36(3):622-631. doi: 10.1002/mds.28355. Epub 2020 Nov 5. Mov Disord. 2021. PMID: 33151010 Free PMC article.
References
-
- Blondel O., Takeda J., Janssen H., Seino S., Bell G.I. 1993. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 268:11356–11363 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

