CD28 exerts protective and detrimental effects in a pulmonary model of paracoccidioidomycosis
- PMID: 20713624
- PMCID: PMC2976317
- DOI: 10.1128/IAI.00297-10
CD28 exerts protective and detrimental effects in a pulmonary model of paracoccidioidomycosis
Abstract
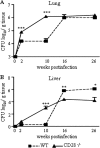
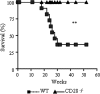
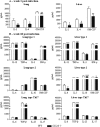
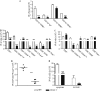
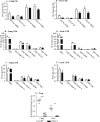
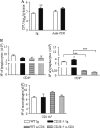
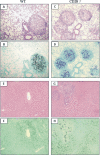
T-cell immunity has been claimed as the main immunoprotective mechanism against Paracoccidioides brasiliensis infection, the most important fungal infection in Latin America. As the initial events that control T-cell activation in paracoccidioidomycosis (PCM) are not well established, we decided to investigate the role of CD28, an important costimulatory molecule for the activation of effector and regulatory T cells, in the immunity against this pulmonary pathogen. Using CD28-deficient (CD28(-/-)) and normal wild-type (WT) C57BL/6 mice, we were able to demonstrate that CD28 costimulation determines in pulmonary paracoccidioidomycosis an early immunoprotection but a late deleterious effect associated with impaired immunity and uncontrolled fungal growth. Up to week 10 postinfection, CD28(-/-) mice presented increased pulmonary and hepatic fungal loads allied with diminished production of antibodies and pro- and anti-inflammatory cytokines besides impaired activation and migration of effector and regulatory T (Treg) cells to the lungs. Unexpectedly, CD28-sufficient mice progressively lost the control of fungal growth, resulting in an increased mortality associated with persistent presence of Treg cells, deactivation of inflammatory macrophages and T cells, prevalent presence of anti-inflammatory cytokines, elevated fungal burdens, and extensive hepatic lesions. As a whole, our findings suggest that CD28 is required for the early protective T-cell responses to P. brasiliensis infection, but it also induces the expansion of regulatory circuits that lately impair adaptive immunity, allowing uncontrolled fungal growth and overwhelming infection, which leads to precocious mortality of mice.
Figures
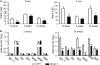

Similar articles
-
Regulatory T cells in paracoccidioidomycosis.Virulence. 2019 Dec;10(1):810-821. doi: 10.1080/21505594.2018.1483674. Epub 2018 Aug 1. Virulence. 2019. PMID: 30067137 Free PMC article. Review.
-
TNF-α and CD8+ T cells mediate the beneficial effects of nitric oxide synthase-2 deficiency in pulmonary paracoccidioidomycosis.PLoS Negl Trop Dis. 2013 Aug 1;7(8):e2325. doi: 10.1371/journal.pntd.0002325. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23936574 Free PMC article.
-
In pulmonary paracoccidioidomycosis IL-10 deficiency leads to increased immunity and regressive infection without enhancing tissue pathology.PLoS Negl Trop Dis. 2013 Oct 24;7(10):e2512. doi: 10.1371/journal.pntd.0002512. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24205424 Free PMC article.
-
Absence of interleukin-4 determines less severe pulmonary paracoccidioidomycosis associated with impaired Th2 response.Infect Immun. 2004 Apr;72(4):2369-78. doi: 10.1128/IAI.72.4.2369-2378.2004. Infect Immun. 2004. PMID: 15039362 Free PMC article.
-
Pulmonary immune responses induced in BALB/c mice by Paracoccidioides brasiliensis conidia.Mycopathologia. 2008 Apr-May;165(4-5):313-30. doi: 10.1007/s11046-007-9072-1. Mycopathologia. 2008. PMID: 18777636 Review.
Cited by
-
Anti-CD25 treatment depletes Treg cells and decreases disease severity in susceptible and resistant mice infected with Paracoccidioides brasiliensis.PLoS One. 2012;7(11):e51071. doi: 10.1371/journal.pone.0051071. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226464 Free PMC article.
-
Regulatory T cells in paracoccidioidomycosis.Virulence. 2019 Dec;10(1):810-821. doi: 10.1080/21505594.2018.1483674. Epub 2018 Aug 1. Virulence. 2019. PMID: 30067137 Free PMC article. Review.
-
Expression of dectin-1 and enhanced activation of NALP3 inflammasome are associated with resistance to paracoccidioidomycosis.Front Microbiol. 2015 Sep 3;6:913. doi: 10.3389/fmicb.2015.00913. eCollection 2015. Front Microbiol. 2015. PMID: 26388856 Free PMC article.
-
NOD-Like Receptor P3 Inflammasome Controls Protective Th1/Th17 Immunity against Pulmonary Paracoccidioidomycosis.Front Immunol. 2017 Jul 10;8:786. doi: 10.3389/fimmu.2017.00786. eCollection 2017. Front Immunol. 2017. PMID: 28740491 Free PMC article.
-
TNF-α and CD8+ T cells mediate the beneficial effects of nitric oxide synthase-2 deficiency in pulmonary paracoccidioidomycosis.PLoS Negl Trop Dis. 2013 Aug 1;7(8):e2325. doi: 10.1371/journal.pntd.0002325. Print 2013. PLoS Negl Trop Dis. 2013. PMID: 23936574 Free PMC article.
References
-
- Acuto, O., and F. Michel. 2003. CD28-mediated co-stimulation: a quantitative support for TCR signaling. Nat. Rev. Immunol. 3:939-951. - PubMed
-
- Arruda, C., C. A. C. Vaz, and V. L. G. Calich. 2007. Aseptic cure of pulmonary paracoccidioidomycosis can be achieved after a previous subcutaneous immunization of susceptible but not resistance mice. Microbes Infect. 9:704-713. - PubMed
-
- Arruda, C., S. S. Kashino, R. A. Fazioli, and V. L. G. Calich. 2007. A primary subcutaneous infection with Paracoccidioides brasiliensis leads to immunoprotection or exacerbated disease depending on the route of challenge. Microbes Infect. 9:308-316. - PubMed
-
- Beck, J. M., M. B. Blackmon, C. M. Rose, S. L. Kimzey, A. M. Preston, and J. M. Green. 2003. T cell costimulatory molecule function determines susceptibility to infection with Pneumocystis carinii in mice. J. Immunol. 171:1969-1977. - PubMed
-
- Belkaid, Y., and K. Tarbell. 2009. Regulatory T cells in the control of host-microorganism interactions. Annu. Rev. Immunol. 27:551-589. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

