CsrA and Cra influence Shigella flexneri pathogenesis
- PMID: 20713625
- PMCID: PMC2976347
- DOI: 10.1128/IAI.00589-10
CsrA and Cra influence Shigella flexneri pathogenesis
Abstract
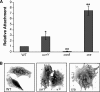
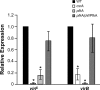
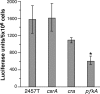
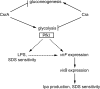
Shigella flexneri is a facultative intracellular pathogen that invades and disrupts the colonic epithelium. In order to thrive in the host, S. flexneri must adapt to environmental conditions in the gut and within the eukaryotic cytosol, including variability in the available carbon sources and other nutrients. We examined the roles of the carbon consumption regulators CsrA and Cra in a cell culture model of S. flexneri virulence. CsrA is an activator of glycolysis and a repressor of gluconeogenesis, and a csrA mutant had decreased attachment and invasion of cultured cells. Conversely, Cra represses glycolysis and activates gluconeogenesis, and the cra mutant had an increase in both attachment and invasion compared to the wild-type strain. Both mutants were defective in plaque formation. The importance of the glycolytic pathway in invasion and plaque formation was confirmed by testing the effect of a mutation in the glycolysis gene pfkA. The pfkA mutant was noninvasive and had cell surface alterations as indicated by decreased sensitivity to SDS and an altered lipopolysaccharide profile. The loss of invasion by the csrA and pfkA mutants was due to decreased expression of the S. flexneri virulence factor regulators virF and virB, resulting in decreased production of Shigella invasion plasmid antigens (Ipa). These data indicate that regulation of carbon metabolism and expression of the glycolysis gene pfkA are critical for synthesis of the virulence gene regulators VirF and VirB, and both the glycolytic and gluconeogenic pathways influence steps in S. flexneri invasion and plaque formation.
Figures

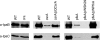
References
-
- Babitzke, P., and T. Romeo. 2007. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 10:156-163. - PubMed
-
- Baker, C. S., I. Morozov, K. Suzuki, T. Romeo, and P. Babitzke. 2002. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol. Microbiol. 44:1599-1610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

