Identification of three novel superantigen-encoding genes in Streptococcus equi subsp. zooepidemicus, szeF, szeN, and szeP
- PMID: 20713629
- PMCID: PMC2976332
- DOI: 10.1128/IAI.00751-10
Identification of three novel superantigen-encoding genes in Streptococcus equi subsp. zooepidemicus, szeF, szeN, and szeP
Abstract
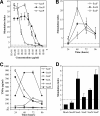
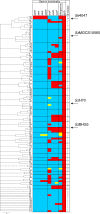
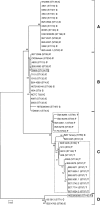
The acquisition of superantigen-encoding genes by Streptococcus pyogenes has been associated with increased morbidity and mortality in humans, and the gain of four superantigens by Streptococcus equi is linked to the evolution of this host-restricted pathogen from an ancestral strain of the opportunistic pathogen Streptococcus equi subsp. zooepidemicus. A recent study determined that the culture supernatants of several S. equi subsp. zooepidemicus strains possessed mitogenic activity but lacked known superantigen-encoding genes. Here, we report the identification and activities of three novel superantigen-encoding genes. The products of szeF, szeN, and szeP share 59%, 49%, and 34% amino acid sequence identity with SPEH, SPEM, and SPEL, respectively. Recombinant SzeF, SzeN, and SzeP stimulated the proliferation of equine peripheral blood mononuclear cells, and tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) production, in vitro. Although none of these superantigen genes were encoded within functional prophage elements, szeN and szeP were located next to a prophage remnant, suggesting that they were acquired by horizontal transfer. Eighty-one of 165 diverse S. equi subsp. zooepidemicus strains screened, including 7 out of 15 isolates from cases of disease in humans, contained at least one of these new superantigen-encoding genes. The presence of szeN or szeP, but not szeF, was significantly associated with mitogenic activity in the S. equi subsp. zooepidemicus population (P < 0.000001, P < 0.000001, and P = 0.104, respectively). We conclude that horizontal transfer of these novel superantigens from and within the diverse S. equi subsp. zooepidemicus population is likely to have implications for veterinary and human disease.
Figures

References
-
- Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403-410. - PubMed
-
- Anzai, T., A. S. Sheoran, Y. Kuwamoto, T. Kondo, R. Wada, T. Inoue, and J. F. Timoney. 1999. Streptococcus equi but not Streptococcus zooepidemicus produces potent mitogenic responses from equine peripheral blood mononuclear cells. Vet. Immunol. Immunopathol. 67:235-246. - PubMed
-
- Arad, G., D. Hillman, R. Levy, and R. Kaempfer. 2001. Superantigen antagonist blocks Th1 cytokine gene induction and lethal shock. J. Leukoc. Biol. 69:921-927. - PubMed
-
- Artiushin, S. C., J. F. Timoney, A. S. Sheoran, and S. K. Muthupalani. 2002. Characterization and immunogenicity of pyrogenic mitogens SePE-H and SePE-I of Streptococcus equi. Microb. Pathog. 32:71-85. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

